Cathepsin B
Cathepsin B belongs to a family of lysosomal cysteine proteases and plays an important role in intracellular proteolysis.[5] In humans, cathepsin B is encoded by the CTSB gene.[6][7] Cathepsin B is upregulated in certain cancers, in pre-malignant lesions, and in various other pathological conditions.[8][9][10][11]
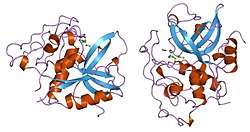
Structure
Gene
The CTSB gene is located at chromosome 8p22, consisting of 13 exons. The promoter of CTSB gene contains a GC-rich region including many SP1 sites, which is similar to housekeeping genes.[12] At least five transcript variants encoding the same protein have been found for this gene.[13]
Protein
Cathepsin B is synthesized on the rough endoplasmic reticulum as a preproenzyme of 339 amino acids with a signal peptide of 17 amino acids.[14][15] Procathepsin B of 43/46 kDa is then transported to the Golgi apparatus, where cathepsin B is formed. Mature cathepsin B is composed of a heavy chain of 25-26 kDa and a light chain of 5kDa, which are linked by a dimer of disulfide.
Function
Cathepsin B may enhance the activity of other proteases, including matrix metalloproteinase, urokinase (serine protease urokinase plasminogen activator), and cathepsin D,[16][17] and thus it has an essential position for the proteolysis of extracellular matrix components, intercellular communication disruption, and reduced protease inhibitor expression.[11] It is also involved in autophagy and catabolism, which is advantageous in tumor malignancy, and it is possibly involved in specific immune resistance.[18]
Clinical significance
Cathepsin B has been proposed as a potentially effective biomarker for a variety of cancers.[16][19][20][21][22][23] Overexpression of cathepsin B is correlated with invasive and metastatic cancers.[24] Cathepsin B is produced in muscle tissue during metabolism.[25] It is capable of crossing the blood-brain barrier[26] and is associated with neurogenesis, specifically in the mouse dentate gyrus. A wide array of diseases result in elevated levels of cathepsin B, which causes numerous pathological processes including cell death, inflammation, and production of toxic peptides. Focusing on neurological diseases, cathepsin B gene knockout studies in an epileptic rodent model have shown cathepsin B causes a significant amount of the apoptotic cell death that occurs as a result of inducing epilepsy.[27] Cathepsin B inhibitor treatment of rats in which a seizure was induced resulted in improved neurological scores, learning ability and much reduced neuronal cell death and pro-apoptotic cell death peptides.[28] Similarly, cathepsin B gene knockout and cathepsin B inhibitor treatment studies in traumatic brain injury mouse models have shown that cathepsin B to be key to causing the resulting neuromuscular dysfunction, memory loss, neuronal cell death and increased production of pro-necrotic and pro-apoptotic cell death peptides.[29][30] In ischemic non-human primate and rodent models, cathepsin B inhibitor treatment prevented a significant loss of brain neurons, especially in the hippocampus.[31][32][33] In a Streptococcus pneumoniae meningitis rodent model, cathepsin B inhibitor treatment greatly improved the clinical course of the infection and reduced brain inflammation and inflammatory Interleukin-1β (IL1-β) and tumor necrosis factor-α (TNF-α).[34] In a transgenic Alzheimer's disease (AD) animal model expressing human amyloid precursor protein (APP) containing the wild-type beta-secretase site sequence found in most AD patients or in guinea pigs, which are a natural model of human wild-type APP processing, genetically deleting the cathepsin B gene or chemically inhibiting cathepsin B brain activity resulted in a significant improvement in the memory deficits that develop in such mice and reduces levels of neurotoxic full-length Abeta(1-40/42) and the particularly pernicious pyroglutamate Abeta(3-40/42), which are thought to cause the disease.[35][36][37][38][39][40][41] In a non-transgenic senescence-accelerated mouse strain, which also has APP containing the wild-type beta-secretase site sequence, treatment with bilobalide, which is an extract of Gingko biloba leaves, also lowered brain Abeta by inhibiting cathepsin B.[42] Moreover, siRNA silencing or chemically inhibiting cathepsin B in primary rodent hippocampal cells or bovine chromaffin cells, which have human wild-type beta-secretase activity, reduces secretion of Abeta by the regulated secretory pathway.[43][44] Mutations in the CTSB gene have been linked to tropical pancreatitis, a form of chronic pancreatitis.[45]
Interactions
Cathepsin B has been shown to interact with:
Cathepsin B is inhibited by:
- Nitroxoline[51]
- CA-074[52]
See also
References
- ENSG00000285132 GRCh38: Ensembl release 89: ENSG00000164733, ENSG00000285132 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000021939 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Sloane BF (April 1990). "Cathepsin B and cystatins: evidence for a role in cancer progression". Seminars in Cancer Biology. 1 (2): 137–52. PMID 2103490.
- Chan SJ, San Segundo B, McCormick MB, Steiner DF (October 1986). "Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs". Proceedings of the National Academy of Sciences of the United States of America. 83 (20): 7721–5. doi:10.1073/pnas.83.20.7721. PMC 386793. PMID 3463996.
- Cao L, Taggart RT, Berquin IM, Moin K, Fong D, Sloane BF (February 1994). "Human gastric adenocarcinoma cathepsin B: isolation and sequencing of full-length cDNAs and polymorphisms of the gene". Gene. 139 (2): 163–9. doi:10.1016/0378-1119(94)90750-1. PMID 8112600.
- Tong B, Wan B, Wei Z, Wang T, Zhao P, Dou Y, Lv Z, Xia Y, Dai Y (September 2014). "Role of cathepsin B in regulating migration and invasion of fibroblast-like synoviocytes into inflamed tissue from patients with rheumatoid arthritis". Clinical and Experimental Immunology. 177 (3): 586–97. doi:10.1111/cei.12357. PMC 4137842. PMID 24749816.
- Lai WF, Chang CH, Tang Y, Bronson R, Tung CH (March 2004). "Early diagnosis of osteoarthritis using cathepsin B sensitive near-infrared fluorescent probes". Osteoarthritis and Cartilage. 12 (3): 239–44. doi:10.1016/j.joca.2003.11.005. PMID 14972341.
- Ha SD, Ham B, Mogridge J, Saftig P, Lin S, Kim SO (January 2010). "Cathepsin B-mediated autophagy flux facilitates the anthrax toxin receptor 2-mediated delivery of anthrax lethal factor into the cytoplasm". The Journal of Biological Chemistry. 285 (3): 2120–9. doi:10.1074/jbc.M109.065813. PMC 2804368. PMID 19858192.
- Yang WE, Ho CC, Yang SF, Lin SH, Yeh KT, Lin CW, Chen MK (2016). "Cathepsin B Expression and the Correlation with Clinical Aspects of Oral Squamous Cell Carcinoma". PLoS One. 11 (3): e0152165. doi:10.1371/journal.pone.0152165. PMC 4816521. PMID 27031837.
- Qian F, Frankfater A, Chan SJ, Steiner DF (April 1991). "The structure of the mouse cathepsin B gene and its putative promoter". DNA and Cell Biology. 10 (3): 159–68. doi:10.1089/dna.1991.10.159. PMID 2012677.
- "Entrez Gene: CTSB cathepsin B".
- Kirschke H, Barrett AJ, Rawlings ND (1995). "Proteinases 1: lysosomal cysteine proteinases". Protein Profile. 2 (14): 1581–643. PMID 8771190.
- Mort JS, Buttle DJ (May 1997). "Cathepsin B". The International Journal of Biochemistry & Cell Biology. 29 (5): 715–20. doi:10.1016/s1357-2725(96)00152-5. PMID 9251238.
- Alapati K, Kesanakurti D, Rao JS, Dasari VR (May 2014). "uPAR and cathepsin B-mediated compartmentalization of JNK regulates the migration of glioma-initiating cells". Stem Cell Research. 12 (3): 716–29. doi:10.1016/j.scr.2014.02.008. PMC 4061617. PMID 24699410.
- Vigneswaran N, Zhao W, Dassanayake A, Muller S, Miller DM, Zacharias W (August 2000). "Variable expression of cathepsin B and D correlates with highly invasive and metastatic phenotype of oral cancer". Human Pathology. 31 (8): 931–7. doi:10.1053/hupa.2000.9035. PMID 10987253.
- Fais S (December 2007). "Cannibalism: a way to feed on metastatic tumors". Cancer Letters. 258 (2): 155–64. doi:10.1016/j.canlet.2007.09.014. PMID 17977647.
- Mirković B, Markelc B, Butinar M, Mitrović A, Sosič I, Gobec S, Vasiljeva O, Turk B, Čemažar M, Serša G, Kos J (August 2015). "Nitroxoline impairs tumor progression in vitro and in vivo by regulating cathepsin B activity". Oncotarget. 6 (22): 19027–42. doi:10.18632/oncotarget.3699. PMC 4662473. PMID 25848918.
- Bian B, Mongrain S, Cagnol S, Langlois MJ, Boulanger J, Bernatchez G, Carrier JC, Boudreau F, Rivard N (May 2016). "Cathepsin B promotes colorectal tumorigenesis, cell invasion, and metastasis". Molecular Carcinogenesis. 55 (5): 671–87. doi:10.1002/mc.22312. PMC 4832390. PMID 25808857.
- Bengsch F, Buck A, Günther SC, Seiz JR, Tacke M, Pfeifer D, von Elverfeldt D, Sevenich L, Hillebrand LE, Kern U, Sameni M, Peters C, Sloane BF, Reinheckel T (September 2014). "Cell type-dependent pathogenic functions of overexpressed human cathepsin B in murine breast cancer progression". Oncogene. 33 (36): 4474–84. doi:10.1038/onc.2013.395. PMC 4139469. PMID 24077280.
- Bao W, Fan Q, Luo X, Cheng WW, Wang YD, Li ZN, Chen XL, Wu D (August 2013). "Silencing of Cathepsin B suppresses the proliferation and invasion of endometrial cancer". Oncology Reports. 30 (2): 723–30. doi:10.3892/or.2013.2496. PMID 23708264.
- Yin M, Soikkeli J, Jahkola T, Virolainen S, Saksela O, Hölttä E (December 2012). "TGF-β signaling, activated stromal fibroblasts, and cysteine cathepsins B and L drive the invasive growth of human melanoma cells". The American Journal of Pathology. 181 (6): 2202–16. doi:10.1016/j.ajpath.2012.08.027. PMID 23063511.
- Ruan J, Zheng H, Rong X, Rong X, Zhang J, Fang W, Zhao P, Luo R (20 February 2016). "Over-expression of cathepsin B in hepatocellular carcinomas predicts poor prognosis of HCC patients". Molecular Cancer. 15: 17. doi:10.1186/s12943-016-0503-9. PMC 4761221. PMID 26896959.
- Vivar C, Potter MC, van Praag H (2012). All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Current Topics in Behavioral Neurosciences. 15. pp. 189–210. doi:10.1007/7854_2012_220. ISBN 978-3-642-36231-6. PMC 4565722. PMID 22847651.
- Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, Duzel E, van Praag H (June 2016). "Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function". Cell Metabolism. 24 (2): 332–40. doi:10.1016/j.cmet.2016.05.025. PMC 6029441. PMID 27345423.
- Houseweart MK, Pennacchio LA, Vilaythong A, Peters C, Noebels JL, Myers RM (September 2003). "Cathepsin B but not cathepsins L or S contributes to the pathogenesis of Unverricht-Lundborg progressive myoclonus epilepsy (EPM1)". Journal of Neurobiology. 56 (4): 315–27. doi:10.1002/neu.10253. PMID 12918016.
- Ni H, Ren SY, Zhang LL, Sun Q, Tian T, Feng X (February 2013). "Expression profiles of hippocampal regenerative sprouting-related genes and their regulation by E-64d in a developmental rat model of penicillin-induced recurrent epilepticus". Toxicology Letters. 217 (2): 162–9. doi:10.1016/j.toxlet.2012.12.010. PMID 23266720.
- Hook GR, Yu J, Sipes N, Pierschbacher MD, Hook V, Kindy MS (March 2014). "The cysteine protease cathepsin B is a key drug target and cysteine protease inhibitors are potential therapeutics for traumatic brain injury". Journal of Neurotrauma. 31 (5): 515–29. doi:10.1089/neu.2013.2944. PMC 3934599. PMID 24083575.
- Luo CL, Chen XP, Yang R, Sun YX, Li QQ, Bao HJ, Cao QQ, Ni H, Qin ZH, Tao LY (October 2010). "Cathepsin B contributes to traumatic brain injury-induced cell death through a mitochondria-mediated apoptotic pathway". Journal of Neuroscience Research. 88 (13): 2847–58. doi:10.1002/jnr.22453. PMID 20653046.
- Yoshida M, Yamashima T, Zhao L, Tsuchiya K, Kohda Y, Tonchev AB, Matsuda M, Kominami E (September 2002). "Primate neurons show different vulnerability to transient ischemia and response to cathepsin inhibition". Acta Neuropathologica. 104 (3): 267–72. doi:10.1007/s00401-002-0554-4. PMID 12172912.
- Tsuchiya K, Kohda Y, Yoshida M, Zhao L, Ueno T, Yamashita J, Yoshioka T, Kominami E, Yamashima T (February 1999). "Postictal blockade of ischemic hippocampal neuronal death in primates using selective cathepsin inhibitors". Experimental Neurology. 155 (2): 187–94. doi:10.1006/exnr.1998.6988. PMID 10072294.
- Tsubokawa T, Yamaguchi-Okada M, Calvert JW, Solaroglu I, Shimamura N, Yata K, Zhang JH (September 2006). "Neurovascular and neuronal protection by E64d after focal cerebral ischemia in rats". Journal of Neuroscience Research. 84 (4): 832–40. doi:10.1002/jnr.20977. PMID 16802320.
- Hoegen T, Tremel N, Klein M, Angele B, Wagner H, Kirschning C, Pfister HW, Fontana A, Hammerschmidt S, Koedel U (November 2011). "The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release". Journal of Immunology. 187 (10): 5440–51. doi:10.4049/jimmunol.1100790. PMID 22003197.
- Hook VY, Kindy M, Hook G (March 2008). "Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein". The Journal of Biological Chemistry. 283 (12): 7745–53. doi:10.1074/jbc.m708362200. PMID 18184658.
- Hook V, Kindy M, Hook G (February 2007). "Cysteine protease inhibitors effectively reduce in vivo levels of brain beta-amyloid related to Alzheimer's disease". Biological Chemistry. 388 (2): 247–52. doi:10.1515/bc.2007.027. PMID 17261088.
- Hook G, Hook VY, Kindy M (September 2007). "Cysteine protease inhibitors reduce brain beta-amyloid and beta-secretase activity in vivo and are potential Alzheimer's disease therapeutics". Biological Chemistry. 388 (9): 979–83. doi:10.1515/BC.2007.117. PMID 17696783.
- Hook VY, Kindy M, Reinheckel T, Peters C, Hook G (August 2009). "Genetic cathepsin B deficiency reduces beta-amyloid in transgenic mice expressing human wild-type amyloid precursor protein". Biochemical and Biophysical Research Communications. 386 (2): 284–8. doi:10.1016/j.bbrc.2009.05.131. PMC 2753505. PMID 19501042.
- Hook G, Hook V, Kindy M (2011). "The cysteine protease inhibitor, E64d, reduces brain amyloid-β and improves memory deficits in Alzheimer's disease animal models by inhibiting cathepsin B, but not BACE1, β-secretase activity". Journal of Alzheimer's Disease. 26 (2): 387–408. doi:10.3233/JAD-2011-110101. PMC 4317342. PMID 21613740.
- Kindy MS, Yu J, Zhu H, El-Amouri SS, Hook V, Hook GR (2012). "Deletion of the cathepsin B gene improves memory deficits in a transgenic ALZHeimer's disease mouse model expressing AβPP containing the wild-type β-secretase site sequence". Journal of Alzheimer's Disease. 29 (4): 827–40. doi:10.3233/JAD-2012-111604. PMC 4309289. PMID 22337825.
- Hook G, Yu J, Toneff T, Kindy M, Hook V (2014). "Brain pyroglutamate amyloid-β is produced by cathepsin B and is reduced by the cysteine protease inhibitor E64d, representing a potential Alzheimer's disease therapeutic". Journal of Alzheimer's Disease. 41 (1): 129–49. doi:10.3233/JAD-131370. PMC 4059604. PMID 24595198.
- Shi C, Zheng DD, Wu FM, Liu J, Xu J (February 2012). "The phosphatidyl inositol 3 kinase-glycogen synthase kinase 3β pathway mediates bilobalide-induced reduction in amyloid β-peptide". Neurochemical Research. 37 (2): 298–306. doi:10.1007/s11064-011-0612-1. PMID 21952928.
- Hook V, Toneff T, Bogyo M, Greenbaum D, Medzihradszky KF, Neveu J, Lane W, Hook G, Reisine T (September 2005). "Inhibition of cathepsin B reduces beta-amyloid production in regulated secretory vesicles of neuronal chromaffin cells: evidence for cathepsin B as a candidate beta-secretase of Alzheimer's disease". Biological Chemistry. 386 (9): 931–40. doi:10.1515/BC.2005.108. PMID 16164418.
- Klein DM, Felsenstein KM, Brenneman DE (March 2009). "Cathepsins B and L differentially regulate amyloid precursor protein processing". The Journal of Pharmacology and Experimental Therapeutics. 328 (3): 813–21. doi:10.1124/jpet.108.147082. PMID 19064719.
- Tandon RK (January 2007). "Tropical pancreatitis". Journal of Gastroenterology. 42 Suppl 17 (Suppl 17): 141–7. doi:10.1007/s00535-006-1930-y. PMID 17238044.
- van der Stappen JW, Williams AC, Maciewicz RA, Paraskeva C (August 1996). "Activation of cathepsin B, secreted by a colorectal cancer cell line requires low pH and is mediated by cathepsin D". International Journal of Cancer. 67 (4): 547–54. doi:10.1002/(SICI)1097-0215(19960807)67:4<547::AID-IJC14>3.0.CO;2-4. PMID 8759615.
- Pavlova A, Björk I (September 2003). "Grafting of features of cystatins C or B into the N-terminal region or second binding loop of cystatin A (stefin A) substantially enhances inhibition of cysteine proteinases". Biochemistry. 42 (38): 11326–33. doi:10.1021/bi030119v. PMID 14503883.
- Estrada S, Nycander M, Hill NJ, Craven CJ, Waltho JP, Björk I (May 1998). "The role of Gly-4 of human cystatin A (stefin A) in the binding of target proteinases. Characterization by kinetic and equilibrium methods of the interactions of cystatin A Gly-4 mutants with papain, cathepsin B, and cathepsin L". Biochemistry. 37 (20): 7551–60. doi:10.1021/bi980026r. PMID 9585570.
- Pol E, Björk I (September 2001). "Role of the single cysteine residue, Cys 3, of human and bovine cystatin B (stefin B) in the inhibition of cysteine proteinases". Protein Science. 10 (9): 1729–38. doi:10.1110/ps.11901. PMC 2253190. PMID 11514663.
- Mai J, Finley RL, Waisman DM, Sloane BF (April 2000). "Human procathepsin B interacts with the annexin II tetramer on the surface of tumor cells". The Journal of Biological Chemistry. 275 (17): 12806–12. doi:10.1074/jbc.275.17.12806. PMID 10777578.
- Hurley EA, Thorley-Lawson DA (December 1988). "B cell activation and the establishment of Epstein-Barr virus latency". The Journal of Experimental Medicine. 168 (6): 2059–75. doi:10.1084/jem.168.6.2059. PMC 2189139. PMID 2848918.
- Murata M, Miyashita S, Yokoo C, Tamai M, Hanada K, Hatayama K, Towatari T, Nikawa T, Katunuma N (March 1991). "Novel epoxysuccinyl peptides. Selective inhibitors of cathepsin B, in vitro". FEBS Letters. 280 (2): 311–15. doi:10.1016/0014-5793(91)80318-w. PMID 2013328.
Further reading
- Yan S, Sloane BF (June 2003). "Molecular regulation of human cathepsin B: implication in pathologies". Biological Chemistry. 384 (6): 845–54. doi:10.1515/BC.2003.095. PMID 12887051.
External links
- The MEROPS online database for peptidases and their inhibitors: C01.060
- Cathepsin+B at the US National Library of Medicine Medical Subject Headings (MeSH)