Capillaria philippinensis
Capillaria philippinensis is a parasitic nematode which causes intestinal capillariasis. This sometimes fatal disease was first discovered in Northern Luzon, Philippines in 1964. Cases have also been reported from China, Egypt, Indonesia, Iran, Japan, Korea, Lao PDR, Taiwan and Thailand.[1] Cases diagnosed in Italy and Spain were believed to be acquired abroad, with one case possibly contracted in Colombia.[2] The natural life cycle of C. philippinensis is believed to involve fish as intermediate hosts, and fish-eating birds as definitive hosts. Humans acquire C. philippinensis by eating small species of infested fish whole and raw.
| Capillaria philippinensis | |
|---|---|
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Nematoda |
| Class: | Enoplea |
| Order: | Enoplida |
| Family: | Capillariidae |
| Genus: | Capillaria |
| Species: | C. philippinensis |
| Binomial name | |
| Capillaria philippinensis Velasquez, Chitwood and Salazar, 1968 | |
Discovery and nomenclature
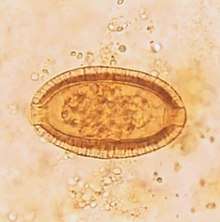
Between the first case reported in 1964 and the end of 1967, more than 1000 cases were documented in and around Northern Luzon particularly at Tagudin, Ilocos Sur, including 77 deaths. Witch doctors were hired by the locals to exorcise the curse placed on them by the river god, which they believed was responsible for this sudden disaster.[3]
In 1968, the cause was identified as Capillaria philippinensis.[4] Adult C. philippinensis are very small, with males measuring 1.5-3.9 mm long and 23-28 µm maximum width, while adult females are 2.3-5.3 mm long and 29-47 µm maximum width. Eggs measure 36-45 µm long and 20 µm wide, and are described as peanut-shaped with a striated shell.
This species has been transferred to the genus Aonchotheca, as Aonchotheca philippinensis,[5] and to the genus Paracapillaria, as Paracapillaria philippinensis.[6] However, this species is almost universally referred to as Capillaria philippinensis in the current medical literature.
Hosts and life cycle

The complete life cycle of C. philippinensis has been demonstrated in experimental studies, and may be either indirect (involving an intermediate host) or direct (complete in one host).[7]
Indirect life cycle. Fish-eating birds which harbor adult C. philippinensis in their intestines, shed embryonated eggs in their feces. When these eggs are fed to uninfected fish, C. philippinensis larvae are recovered from the intestines of fish. If the fish are fed to uninfested birds, the larvae develop into adults in the intestinal tract of the birds. Larvae recovered from the fish also developed into adults when fed to gerbils or monkeys, with eggs shed in the feces of these mammalian hosts. Naturally infested fish (Hypseleotris bipartita and Apagon sp.) and birds (Ixobrychus sp.) have also been found. Humans become infested when they eat raw or undercooked fish, probably small fish eaten whole, which have the infective larvae in their intestinal tract. Raw fish are commonly eaten by several of the Asian cultures in which C. philippinensis infestations have been found.
While the natural host range is not known, experimental infestations of several fishes, including Cyprinus carpio, Puntius gonionotus, Rasbora borapetensis, Eleotris melanosoma, Ambassis commersoni and Apogon sp., with C. philippinensis eggs yielded infective larvae. Experimental infestations with larvae of several birds, including Amaurornis phoenicurus, Ardeola bacchus, Nycticorax nycticorax, Bubulcus ibis, Ixobrychus sinensis, Gallinula chloropus, and Rostratula benghalensis yielded mature adults.
Direct life cycle. Researchers also found that feeding just a few dozen larvae from the intestines of fish to Mongolian gerbils (Meriones unguiculatus) or monkeys (Macaca sp.) led to infestations with thousands of adult worms through "autoinfection". Autoinfection is when the offspring produced by adults can reinfest the same host, allowing the infestation to multiply within a single host animal. Both oviparous (egg-laying) and larviparous (giving birth to active larvae) adult female C. philippinensis were found in Mongolian gerbils and some birds. The experimentally infested monkeys never developed any clinical symptoms, even during prolonged, active infestations. Of several rodents tested, only Mongolian gerbils developed severe symptoms due to infestation and died.
Pathology
Although C. philippinensis infections are rare, it can serve as an indicator that one is being exposed to raw or undercooked fish. Early diagnosis of the parasite is beneficial so the number of worms in an infected person would not increase.[8]
Worms create infection by penetrating the mucosa of the small intestine and reentering the lumen. As they progress into the body, they cause the mucosa and submucosa to degenerate. Infected people can have abdominal pains, diarrhea, weight loss, weakness, malaise, anorexia, and emaciation. They also experience loss of proteins and electrolytes and malabsorption of fats and sugars. If symptoms and the number of worms increase, it can eventually lead to death.
Diagnosis
This parasite can be diagnosed by taking a tissue biopsy from the small intestine or by examining stool samples through a microscope.[9] In a heavily infected person, it is best to examine their feces because it will show an abundance of adult worms and eggs. When looking at the eggs of C. philippinensis, one must be able to distinguish it from the eggs of Trichuris trichiura. C. philippinensis eggs have nonprotruding polar plugs and are slightly smaller than Trichuris trichiura eggs.[10]
Treatment
C. philippinensis infections should be treated with 200 mg of mebendazole. This drug is taken twice a day for 20 days or until all symptoms subside and there are no longer eggs present in the stool samples of the patient. Another drug that may be used is albendazole 400 mg, which is taken orally every day for at least 10 days.[11]
References
- Limsrivilai, Julajak; Pongprasobchai, Supot; Apisarnthanarak, Piyaporn; Manatsathit, Sathaporn (2014). "Intestinal capillariasis in the 21st century: clinical presentations and role of endoscopy and imaging". BMC Gastroenterology. 14 (1): 207. doi:10.1186/s12876-014-0207-9. ISSN 1471-230X. PMC 4271459. PMID 25492259.

- Dronda; Chaves, F; Sanz, A; Lopez-Velez, R (1993). "Human intestinal capillariasis in an area of nonendemicity: case report and review". Clinical Infectious Diseases. 17 (5): 909–12. doi:10.1093/clinids/17.5.909. PMID 8286640.
- Cross, J H (1992). "Intestinal capillariasis". Clinical Microbiology Reviews. 5 (2): 120–9. doi:10.1128/cmr.5.2.120. PMC 358231. PMID 1576584.
- Chitwood; Valesquez, C; Salazar, NG (1968). "Capillaria philippinensis sp. n. (Nematoda: Trichinellida), from the intestine of man in the Philippines". Journal of Parasitology. 54 (2): 368–71. doi:10.2307/3276953. JSTOR 3276953. PMID 5647122.
- Moravec (1982). "Proposal of a new systematic arrangement of nematodes of the family Capillariidae". Folia Parasitologica. 29 (2): 119–32. PMID 7106653.
- Moravec (2001). "Redescription and systematic status of Capillaria philippinensis, an intestinal parasite of human beings". Journal of Parasitology. 87 (1): 161–4. doi:10.2307/3285194. JSTOR 3285194. PMID 11227884.
- Saichua; Nithikathkul, C; Kaewpitoon, N (2008). "Human intestinal capillariasis in Thailand". World Journal of Gastroenterology. 14 (4): 506–10. doi:10.3748/wjg.14.506. PMC 2681139. PMID 18203280.
- Baron, Ellen Jo; Jorgensen, James H.; Landry, Marie Louise (2007). Manual of Clinical Microbiology [C. philippinensis] (9 ed.). Washington, DC, USA: ASM Press. pp. 2191–2192. doi:10.1086/524076.
- "Capillariasis FAQs". www.CDC.gov. Centers for Disease Control and Prevention. January 10, 2012. Retrieved 9 December 2013.
- Roberts, Larry; Janovy, John; Nadler, Steve (2012). Foundations of Parasitology (9 ed.). New York: McGraw-Hill Education. p. 381. ISBN 978-0073524191.
- Warrell, David A.; Cox, Timothy M.; Firth, John D., eds. (2005). Oxford Textbook of Medicine (4 ed.). New York: Oxford University Press Inc. p. 808. doi:10.1093/fampra/cmg441.