Bovine malignant catarrhal fever
Bovine malignant catarrhal fever (BMCF) is a fatal lymphoproliferative disease[1] caused by a group of ruminant gamma herpes viruses including Alcelaphine gammaherpesvirus 1 (AlHV-1)[2] and Ovine gammaherpesvirus 2 (OvHV-2)[1][3] These viruses cause unapparent infection in their reservoir hosts (sheep with OvHV-2 and wildebeest with AlHV-1), but are usually fatal in cattle and other ungulates such as deer, antelope, and buffalo.[2] In Southern Africa the disease is known as snotsiekte, from the Afrikaans.[4][5]
| Alcelaphine gammaherpesvirus 1 (AlHV-1), Ovine gammaherpesvirus 2 (OHV-2) | |
|---|---|
| Virus classification | |
| (unranked): | Virus |
| Realm: | Duplodnaviria |
| Kingdom: | Heunggongvirae |
| Phylum: | Peploviricota |
| Class: | Herviviricetes |
| Order: | Herpesvirales |
| Family: | Herpesviridae |
| Genus: | Macavirus |
| Species: | Alcelaphine gammaherpesvirus 1 (AlHV-1), Ovine gammaherpesvirus 2 (OHV-2) |
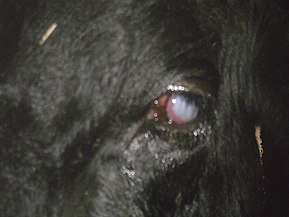
BMCF is an important disease where reservoir and susceptible animals mix. There is a particular problem with Bali cattle in Indonesia,[6] bison in the US[7] and in pastoralist herds in Eastern and Southern Africa.[8][9]
Disease outbreaks in cattle are usually sporadic although infection of up to 40% of a herd has been reported. The reasons for this are unknown. Some species appear to be particularly susceptible, for example Pére David's deer,[10] Bali cattle[6] and bison,[7] with many deer dying within 48 hours of the appearance of the first symptoms and bison within three days.[1][11] In contrast, post infection cattle will usually survive a week or more.[12]
Epidemiology
The term bovine malignant catarrhal fever has been applied to three different patterns of disease:
- In Africa, wildebeests carry a lifelong infection of AlHV-1 but are not affected by the disease.[2] The virus is passed from mother to offspring and shed mostly in the nasal secretions of wildebeest calves under one year old.[13][14] Wildebeest associated MCF is transmitted from wildebeest to cattle normally following the wildebeest calving period. Cattle of all ages are susceptible to the disease, with a higher infection rate in adults, particularly in peripartuent females.[15] Cattle are infected by contact with the secretions, but do not spread the disease to other cattle. Because no commercial treatment or vaccine is available for this disease, livestock management is the only method of control. This involves keeping cattle away from wildebeest during the critical calving period. This results in Massai pastoralists in Tanzania and Kenya being excluded from prime pasture grazing land during the wet season leading to a loss in productivity.[16] In Eastern and Southern Africa MCF is classed as one of the five most important problems affecting pastoralists along with East coast fever, contagious bovine pleuropneumonia, foot and mouth disease and anthrax.[17] Hartebeests and topi also may carry the disease.[18] However, hartebeests and other antelopes are infected by a variant, Alcelaphine herpesvirus 2.
- Throughout the rest of the world, cattle and deer contract BMCF by close contact with sheep or goats during lambing. The natural host reservoir for Ovine herpesvirus 2 is the subfamily Caprinae (sheep and goats) whilst MCF affected animals are from the families Bovidae, Cervidae and suidae.[19][20] Susceptibility to OHV-2 varies by species, with domestic cattle and zebus somewhat resistant, water buffalo and most deer somewhat susceptible, and bison, Bali cattle, and Père David's deer very susceptible.[21] OHV-2 viral DNA has been detected in the alimentary, respiratory and urino-genital tracts of sheep[22] all of which could be possible transmission routes. Antibody from sheep and from cattle with BMCF is cross reactive with AlHV-1.[18]
- AHV-1/OHV-2 can also cause problems in zoological collections, where inapparently infected hosts (wildebeest and sheep) and susceptible hosts are often kept in close proximity.[23]
- Feedlot bison in North America not in contact with sheep have also been diagnosed with a form of BMCF. OHV-2 has been recently documented to infect herds of up to 5 km away from the nearest lambs, with the levels of infected animals proportional to the distance away from the closest herds of sheep.[24]
The incubation period of BMCF is not known, however intranasal challenge with AHV-1 induced MCF in one hundred percent of challenged cattle between 2.5 and 6 weeks.[25] Shedding of the virus is greater from 6–9 month old lambs than from adults.[1] After experimental infection of sheep, there is limited viral replication in nasal cavity in the first 24 hours after infection, followed by later viral replication in other tissues.[1]
Clinical signs
The most common form of the disease is the head and eye form. Typical symptoms of this form include fever, depression, discharge from the eyes and nose, lesions of the buccal cavity and muzzle, swelling of the lymph nodes, opacity of the corneas leading to blindness, inappetence and diarrhea. Some animals have neurologic signs, such as ataxia, nystagmus, and head pressing. Animals that become infected with the virus can become extremely sensitive to touch, especially around the head. It is also possible that become aggressive and charge at approaching animals and people. If the virus continues untreated, seizures could develop. Affected animal usually die five to ten days of the first signs of clinical signs. Once the cow shows clinical signs there is no chance of recovering.[26]
Peracute, alimentary and cutaneous clinical disease patterns have also been described.[27] Death usually occurs within ten days.[28] The mortality rate in symptomatic animals is 90 to 100 percent.[21] Treatment is supportive only.
Factors
There are many factors that can increase the chances of infection or affect the severity of an outbreak. The amount of animals in the herd, population density and species of the susceptible hosts are huge factors. Other factors include closeness of contact and amount of virus available for transmission.[29]
Diagnosis
Diagnosis of BMCF depends on a combination of history and symptoms, histopathology[27] and detection in the blood or tissues of viral antibodies by ELISA[30][31] or of viral DNA by PCR.[22][32][33] The characteristic histologic lesions of MCF are lymphocytic arteritis with necrosis of the blood vessel wall and the presence of large T lymphocytes mixed with other cells.[1] The similarity of MCF clinical signs to other enteric diseases, for example blue tongue, mucosal disease and foot and mouth make laboratory diagnosis of MCF important.[34] The world organisation for animal health[27] recognises histopathology as the definitive diagnostic test, but laboratories have adopted other approaches with recent developments in molecular virology. No vaccine has as yet been developed.
Prognosis
Bovine malignant catarrhal fever usually is fatal in susceptible species like cattle and bison, and any animal that survives will remain infectious for the rest of its life even if it shows no subsequent signs of the disease. Such survivors may relapse and suffer attacks in later life, but what is of more practical importance is that animals with latent infections may be unrecognised carriers that cause unexplained cases. This possibility must be borne in mind when seeking the source of mysterious outbreaks.[29]
Vaccine
Unfortunately a vaccine for malignant catarrhal fever (MCF) has not yet been developed.[1] Developing a vaccine has been difficult because the virus will not grow in cell culture and until recently it was not known why. Researchers at the Agricultural Research Service (ARS) found that the virus undergoes changes within the animal's body, a process known as "cell tropism switching". In cell tropism switching, the virus targets different cells at different points in its life cycle. This phenomenon explains why it has been impossible to grow the virus on any one particular cell culture.
Because the virus is transmitted from sheep to bison and cattle, researchers are first focusing on the viral life cycle in sheep. The viral life cycle is outlined in three stages: entry, maintenance, and shedding. Entry occurs through the sheep's nasal cavity and enters into the lungs where it replicates. The virus undergoes a tropic change and infects lymphocytes, also known as white blood cells, which play a role in the sheep's immune system. In the maintenance stage the virus remains on the sheep's lymphocytes and circulates the body. Finally, during the shedding stage, the virus undergoes another change and shifts its target cells from lymphocytes to nasal cavity cells, where it is then shed through nasal secretions.[35] This discovery undoubtedly puts scientists on the right track for developing a vaccine – starting with the correct cell culture for each stage of the virus lifecycle – but ARS researchers are also looking into alternative methods to develop a vaccine. Researchers are experimenting with the MCF virus that infects topi (an African antelope) because it will grow in cell culture and does not infect cattle. Researchers hope that inserting genes from the sheep MCF virus into the topi MCF virus will ultimately be an effective MCF vaccine for cattle and bison.[35] While there is much ground left to cover, scientists are getting closer and closer to developing a vaccine.
References
- o'Toole, D.; Li, H. (2014). "The Pathology of Malignant Catarrhal Fever, with an Emphasis on Ovine Herpesvirus 2". Veterinary Pathology. 51 (2): 437–452. doi:10.1177/0300985813520435. PMID 24503439.
- Plowright, W.; Ferris, R. D.; Scott, G. R. (1960). "Blue Wildebeest and the Ætiological Agent of Bovine Malignant Catarrhal Fever". Nature. 188 (4757): 1167–1169. Bibcode:1960Natur.188.1167P. doi:10.1038/1881167a0. PMID 13736396.
- Schultheiss, Patricia C.; Collins, James K.; Spraker, Terry R.; Demartini, James C. (2000). "Epizootic Malignant Catarrhal Fever in Three Bison Herds: Differences from Cattle and Association with Ovine Herpesvirus-2". Journal of Veterinary Diagnostic Investigation. 12 (6): 497–502. doi:10.1177/104063870001200602. PMID 11108448.
- Elizabeth S. Williams; Ian K. Barker (28 February 2008). Infectious Diseases of Wild Mammals. John Wiley & Sons. pp. 157–. ISBN 978-0-470-34481-1.
- Lee Merriam Talbot; Martha H. Talbot (1963). The Wildebeest in Western Masailand, East Africa. National Academies. pp. 52–. NAP:13180.
- Wiyono, A.; Baxter, S. I.; Saepulloh, M.; Damayanti, R.; Daniels, P.; Reid, H. W. (1994). "PCR detection of ovine herpesvirus-2 DNA in Indonesian ruminants--normal sheep and clinical cases of malignant catarrhal fever". Veterinary Microbiology. 42 (1): 45–52. doi:10.1016/0378-1135(94)90076-0. PMID 7839584.
- Berezowski, John Andrew; Appleyard, Greg D.; Crawford, Timothy B.; Haigh, Jerry; Li, Hong; Middleton, Dorothy M.; O'Connor, Brendan P.; West, Keith; Woodbury, Murray (2005). "An Outbreak of Sheep-Associated Malignant Catarrhal Fever in Bison (Bison Bison) after Exposure to Sheep at a Public Auction Sale". Journal of Veterinary Diagnostic Investigation. 17 (1): 55–58. doi:10.1177/104063870501700110. PMID 15690951.
- Cleaveland S; Kusiluka L; Ole Kuwai J; Bell C; Kazwala R. 2001. "Assessing the impact of Malignant Catarrhal Fever in Ngorongoro District, Tanzania. A study commissioned by the Animal Health Programme, DFID". Cite journal requires
|journal=(help)CS1 maint: uses authors parameter (link) - Bedelian, Claire; Nkedianye, David; Herrero, Mario (2007). "Maasai perception of the impact and incidence of malignant catarrhal fever (MCF) in southern Kenya". Preventive Veterinary Medicine. 78 (3–4): 296–316. doi:10.1016/j.prevetmed.2006.10.012. PMID 17123651.
- Orr, M.B.; MacKintosh, C.G. (1988). "An outbreak of malignant catarrhal fever in Père David's deer (Elaphurus davidianus)". New Zealand Veterinary Journal. 36 (1): 19–21. doi:10.1080/00480169.1988.35466. PMID 16031426.
- O'Toole, D.; Li, H.; Sourk, C.; Montgomery, D. L.; Crawford, T. B. (2002). "Malignant Catarrhal Fever in a Bison (Bison Bison) Feedlot, 1993–2000". Journal of Veterinary Diagnostic Investigation. 14 (3): 183–193. doi:10.1177/104063870201400301. PMID 12033673.
- Holliman, A.; Daniel, R.; Twomey, D. F.; Barnett, J.; Scholes, S.; Willoughby, K.; Russell, G. (2007). "Malignant catarrhal fever in cattle in the UK". Veterinary Record. 161 (14): 494–495. doi:10.1136/vr.161.14.494-e. PMID 17921444.
- Mushi, E. Z.; Rurangirwa, F. R. (1981). "Malignant catarrhal fever virus shedding by infected cattle". Bulletin of Animal Health and Production in Africa. Bulletin des Sante et Production Animales en Afrique. 29 (1): 111–2. PMID 7296019.
- Baxter, S. I.; Wiyono, A.; Pow, I.; Reid, H. W. (1997). "Identification of Ovine Herpes Virus-2 infection in sheep". Archives of Virology. 142 (4): 823–831. doi:10.1007/s007050050121. PMID 9170507.
- Barnard, B. J.; Van der Lugt, J. J.; Mushi, E. Z. (1994). "Malignant Catarrhal Fever". In Coetzer, J. A. W.; Thompson, G. R.; Tustin, R. C. (eds.). Infectious Diseases of Livestock. New York: Oxford University Press. ISBN 978-0-19-570506-5.
- Homewood, K. H.; Rodgers, W. A.; Arhem, K. (1987). "Ecology of pastoralism in Ngorongoro Conservation Area, Tanzania". The Journal of Agricultural Science. 108: 47–72. doi:10.1017/S0021859600064133.
- Boone, R. B.; Coughenour, M. B. (2001). A system for integrated management and assessment of east African pastoral lands. Balancing food security, wildlife conservation, and ecosystem integrity. Final report to the Global Livestock Collaborative Research Support Program (Report).
- Fenner, Frank J.; Gibbs, E.; Paul, J.; Murphy, Frederick A.; Rott, Rudolph; Studdert, Michael J.; White, David O. (1993). Veterinary Virology (2nd ed.). Academic Press. ISBN 978-0-12-253056-2.
- O'Toole, D.; Taus, N. S.; Montgomery, D. L.; Oaks, J. L.; Crawford, T. B.; Li, H. (2007). "Intra-nasal Inoculation of American Bison (Bison bison) with Ovine Herpesvirus-2 (OvHV-2) Reliably Reproduces Malignant Catarrhal Fever". Veterinary Pathology. 44 (5): 655–662. doi:10.1354/vp.44-5-655. PMID 17846237.
- Taus, N. S.; Herndon, D. R.; Traul, D. L.; Stewart, J. P.; Ackermann, M.; Li, H.; Knowles, D. P.; Lewis, G. S.; Brayton, K. A. (2007). "Comparison of ovine herpesvirus 2 genomes isolated from domestic sheep (Ovis aries) and a clinically affected cow (Bos bovis)". Journal of General Virology. 88 (Pt 1): 40–45. doi:10.1099/vir.0.82285-0. PMID 17170434.
- "Malignant Catarrhal fever" (PDF). The Center for Food Security and Public Health at Iowa State University. 2005. Retrieved 2006-05-13.
- Hussy, D.; Stauber, N.; Leutenegger, C. M.; Rieder, S.; Ackermann, M. (2001). "Quantitative Fluorogenic PCR Assay for Measuring Ovine Herpesvirus 2 Replication in Sheep". Clinical and Vaccine Immunology. 8 (1): 123–128. doi:10.1128/CDLI.8.1.123-128.2001. PMC 96020. PMID 11139205.
- Cooley, A. Jim; Taus, Naomi S.; Li, Hong (2008). "Development of a Management Program for a Mixed Species Wildlife Park Following an Occurrence of Malignant Catarrhal Fever". Journal of Zoo and Wildlife Medicine. 39 (3): 380–385. doi:10.1638/2007-0181.1. PMID 18817000.
- Li, H.; Karney, G.; O'Toole, D.; Crawford, T. B. (2008). "Long distance spread of malignant catarrhal fever virus from feedlot lambs to ranch bison". The Canadian Veterinary Journal. 49 (2): 183–5. PMC 2216446. PMID 18309750.
- Haig, David M.; Grant, Dawn; Deane, David; Campbell, Iris; Thomson, Jackie; Jepson, Catherine; Buxton, David; Russell, George C. (2008). "An immunisation strategy for the protection of cattle against alcelaphine herpesvirus-1-induced malignant catarrhal fever". Vaccine. 26 (35): 4461–4468. doi:10.1016/j.vaccine.2008.06.056. PMID 18601965.
- "NADIS Animal Health Skills - Malignant Catarrhal Fever (MCF)". www.nadis.org.uk. Retrieved 2019-04-02.
- OIE. OIE Manual of Diagnostic Tests and Vaccines for terrestrial Animal (5th ed.). France. pp. 570–579.
- Carter, G.R.; Flores, E.F.; Wise, D.J. (2006). "Herpesviridae". A Concise Review of Veterinary Virology. Retrieved 2006-06-10.
- "Overview of Malignant Catarrhal Fever - Generalized Conditions". Merck Veterinary Manual. Retrieved 2019-04-02.
- Fraser, S.J.; Nettleton, P.F.; Dutia, B.M; Haig, D.M.; Russell, G.C. (2006). "Development of an enzyme-linked immunosorbent assay for the detection of antibodies against malignant catarrhal fever viruses in cattle serum". Veterinary Microbiology. 116 (1–3): 21–28. doi:10.1016/j.vetmic.2006.03.002. PMID 16621342.
- Li, Hong; McGuire, Travis C.; Müller-Doblies, Uwe U.; Crawford, Timothy B. (2001). "A Simpler, More Sensitive Competitive Inhibition Enzyme-Linked Immunosorbent Assay for Detection of Antibody to Malignant Catarrhal Fever Viruses". Journal of Veterinary Diagnostic Investigation. 13 (4): 361–364. doi:10.1177/104063870101300417. PMID 11478614.
- Cunha, C. W.; Otto, L.; Taus, N. S.; Knowles, D. P.; Li, H. (2009). "Development of a Multiplex Real-Time PCR for Detection and Differentiation of Malignant Catarrhal Fever Viruses in Clinical Samples". Journal of Clinical Microbiology. 47 (8): 2586–2589. doi:10.1128/JCM.00997-09. PMC 2725674. PMID 19494077.
- Traul, Donald L.; Taus, Naomi S.; Oaks, J. Lindsay; Toole, Donal O'; Rurangirwa, Fred R.; Baszler, Timothy V.; Li, Hong (2007). "Validation of Nonnested and Real-Time PCR for Diagnosis of Sheep-Associated Malignant Catarrhal Fever in Clinical Samples". Journal of Veterinary Diagnostic Investigation. 19 (4): 405–408. doi:10.1177/104063870701900412. PMID 17609352.
- Bexiga, R.; Guyot, H.; Saegerman, C.; Mauroy, A.; Rollin, F.; Thiry, E.; Philbey, A. W.; Logue, D. N.; Mellor, D. J.; Barrett, D. C.; Ellis, K. (2007). "Clinical differentiation of malignant catarrhal fever, mucosal disease and bluetongue". The Veterinary Record. 161 (25): 858–9. PMID 18156595.
- "Figuring Out Puzzling Animal Diseases". USDA Agricultural Research Service. 2010-04-02. Archived from the original on 2010-04-05. Retrieved July 9, 2017.
External links
| Wikimedia Commons has media related to Bovine malignant catarrhal fever. |
- Current status of Bovine malignant catarrhal fever worldwide at OIE. WAHID Interface - OIE World Animal Health Information Database
- Disease card