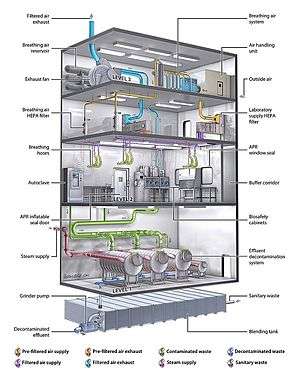
Biosafety level
A biosafety level (BSL), or pathogen/protection level, is a set of biocontainment precautions required to isolate dangerous biological agents in an enclosed laboratory facility. The levels of containment range from the lowest biosafety level 1 (BSL-1) to the highest at level 4 (BSL-4). In the United States, the Centers for Disease Control and Prevention (CDC) have specified these levels.[2] In the European Union, the same biosafety levels are defined in a directive.[3] In Canada the four levels are known as Containment Levels.[4] Facilities with these designations are also sometimes given as P1 through P4 (for pathogen or protection level), as in the term P3 laboratory.[5]
At the lowest level of biosafety, precautions may consist of regular hand-washing and minimal protective equipment. At higher biosafety levels, precautions may include airflow systems, multiple containment rooms, sealed containers, positive pressure personnel suits, established protocols for all procedures, extensive personnel training, and high levels of security to control access to the facility.
History
The first prototype Class III (maximum containment) biosafety cabinet was fashioned in 1943 by Hubert Kaempf Jr., then a U.S. Army soldier, under the direction of Arnold G. Wedum, Director (1944–69) of Industrial Health and Safety at the United States Army Biological Warfare Laboratories, Camp Detrick, Maryland. Kaempf was tired of his MP duties at Detrick and was able to transfer to the sheet metal department working with the contractor, the H.K. Ferguson Co.[6]
On 18 April 1955, fourteen representatives met at Camp Detrick in Frederick, Maryland. The meeting was to share knowledge and experiences regarding biosafety, chemical, radiological, and industrial safety issues that were common to the operations at the three principal biological warfare (BW) laboratories of the U.S. Army.[7] Because of the potential implication of the work conducted at biological warfare laboratories, the conferences were restricted to top level security clearances. Beginning in 1957, these conferences were planned to include non-classified sessions as well as classified sessions to enable broader sharing of biological safety information. It was not until 1964, however, that conferences were held in a government installation not associated with a biological warfare program.[8]
Over the next ten years, the biological safety conferences grew to include representatives from all federal agencies that sponsored or conducted research with pathogenic microorganisms. By 1966 it began to include representatives from universities, private laboratories, hospitals, and industrial complexes. Throughout the 1970s, participation in the conferences continued to expand and by 1983 discussions began regarding the creation of a formal organization.[8] The American Biological Safety Association (ABSA) was officially established in 1984 and a constitution and bylaws were drafted the same year. As of 2008, ABSA includes some 1,600 members in its professional association.[8]
In 1977 Jim Peacock of the Australian Academy of Science asked Bill Snowdon, then Chief CSIRO AAHL if he could have the newly released USA NIH and the British equivalent requirements for the development of infrastructure for bio-containment reviewed by AAHL personnel with a view to recommending the adoption of one of them by Australian authorities. The review was carried out by CSIRO AAHL Project Manager Bill Curnow and CSIRO Engineer Arthur Jenkins. They drafted outcomes for each of the levels of security. AAHL was notionally classified as "substantially beyond P4". These were adopted by the Australian Academy of Science and became the basis for Australian Legislation. It opened in 1985 costing $185 million, built on Corio Oval.[9] The Australian Animal Health Laboratory is a Class 4/ P4 Laboratory.
Levels
Biosafety level 1
Biosafety level 1 (BSL-1) is suitable for work with well-characterized agents which do not cause disease in healthy humans. In general, these agents should pose minimal potential hazard to laboratory personnel and the environment.[10] At this level, precautions are limited relative to other levels. Laboratory personnel must wash their hands upon entering and exiting the lab. Research with these agents may be performed on standard open laboratory benches without the use of special containment equipment. However, eating and drinking are generally prohibited in laboratory areas.[10] Potentially infectious material must be decontaminated before disposal, either by adding a chemical such as bleach or isopropanol or by packaging for decontamination elsewhere.[10] Personal protective equipment is only required for circumstances where personnel might be exposed to hazardous material.[10] BSL-1 laboratories must have a door which can be locked to limit access to the lab. However, it is not necessary for BSL-1 labs to be isolated from the general building.[11]
This level of biosafety is appropriate for work with several kinds of microorganisms including non-pathogenic strains of Escherichia coli and Staphylococcus, Bacillus subtilis, Saccharomyces cerevisiae and other organisms not suspected to contribute to human disease.[12] Due to the relative ease and safety of maintaining a BSL-1 laboratory, these are the types of laboratories generally used as teaching spaces for high schools and colleges.[11]
Biosafety level 2
At this level, all precautions used at Biosafety Level 1 are followed, and some additional precautions are taken. BSL-2 differs from BSL-1 in that:
- Laboratory personnel have specific training in handling pathogenic agents and are directed by scientists with advanced training.
- Access to the laboratory is limited when work is being conducted.
- Extreme precautions are taken with contaminated sharp items.
- Certain procedures in which infectious aerosols or splashes may be created are conducted in biological safety cabinets or other physical containment equipment.[10]
Biosafety level 2 is suitable for work involving agents of moderate potential hazard to personnel and the environment.[11] This includes various microbes that cause mild disease to humans, or are difficult to contract via aerosol in a lab setting.[13] Examples include Hepatitis A, B, and C viruses, human immunodeficiency virus (HIV), pathogenic strains of Escherichia coli and Staphylococcus, Salmonella, Plasmodium falciparum, and Toxoplasma gondii.[13][14]
Biosafety level 3

Biosafety level 3 is appropriate for work involving microbes which can cause serious and potentially lethal disease via the inhalation route.[10] This type of work can be done in clinical, diagnostic, teaching, research, or production facilities.[11] Here, the precautions undertaken in BSL-1 and BSL-2 labs are followed, as well as additional measures including:
- All laboratory personnel are provided medical surveillance and offered relevant immunizations (where available) to reduce the risk of an accidental or unnoticed infection.[10]
- All procedures involving infectious material must be done within a biological safety cabinet.[10]
- Laboratory personnel must wear solid-front protective clothing (i.e. gowns that tie in the back). This cannot be worn outside of the laboratory and must be discarded or decontaminated after each use.[10]
- A laboratory-specific biosafety manual must be drafted which details how the laboratory will operate in compliance with all safety requirements.[10]
In addition, the facility which houses the BSL-3 laboratory must have certain features to ensure appropriate containment. The entrance to the laboratory must be separated from areas of the building with unrestricted traffic flow.[10] Additionally, the laboratory must be behind two sets of self-closing doors (to reduce the risk of aerosols escaping).[11] The construction of the laboratory is such that it can be easily cleaned. Carpets are not permitted, and any seams in the floors, walls, and ceilings are sealed to allow for easy cleaning and decontamination.[10] Additionally, windows must be sealed, and a ventilation system installed which forces air to flow from the "clean" areas of the lab to the areas where infectious agents are handled.[10] Air from the laboratory must be filtered before it can be recirculated.[10]
Biosafety level 3 is commonly used for research and diagnostic work involving various microbes which can be transmitted by aerosols and/or cause severe disease. These include Francisella tularensis, Mycobacterium tuberculosis, Chlamydia psittaci, Venezuelan equine encephalitis virus, Eastern equine encephalitis virus, SARS-CoV-1, SARS-CoV-2, MERS-CoV, Coxiella burnetii, Rift Valley fever virus, Rickettsia rickettsii, several species of Brucella, chikungunya, yellow fever virus, West Nile virus, Yersinia pestis.[14][15]
China has, as of June 2020, "more than 20" BSL-3 laboratories as part of the Chinese Center for Disease Control and Prevention, including at least one mobile unit. In response to the COVID-19 pandemic in China, authorities in May "unveiled a plan... that requires every province to establish at least one BSL-3 lab, and major cities to have at least one BSL-2 lab."[16]
Biosafety level 4

Biosafety level 4 (BSL-4) is the highest level of biosafety precautions, and is appropriate for work with agents that could easily be aerosol-transmitted within the laboratory and cause severe to fatal disease in humans for which there are no available vaccines or treatments.[10] BSL-4 laboratories are generally set up to be either cabinet laboratories or protective-suit laboratories.[10] In cabinet laboratories, all work must be done within a class III biosafety cabinet.[10] Materials leaving the cabinet must be decontaminated by passing through an autoclave or a tank of disinfectant.[10] The cabinets themselves are required to have seamless edges to allow for easy cleaning. Additionally the cabinet and all materials within must be free of sharp edges in order to reduce the risk of damage to the gloves.[10] In a protective-suit laboratory, all work must be done in a class II biosafety cabinet by personnel wearing a positive pressure suit.[10] In order to exit the BSL-4 laboratory, personnel must pass through a chemical shower for decontamination, then a room for removing the positive-pressure suit, followed by a personal shower.[10] Entry into the BSL-4 laboratory is restricted to trained and authorized individuals, and all persons entering and exiting the laboratory must be recorded.[10]
As with BSL-3 laboratories, BSL-4 laboratories must be separated from areas that receive unrestricted traffic. Additionally airflow is tightly controlled to ensure that air always flows from "clean" areas of the lab to areas where work with infectious agents is being performed.[10] The entrance to the BSL-4 lab must also employ airlocks to minimize the possibility that aerosols from the lab could be removed from the lab. All laboratory waste, including filtered air, water, and trash must also be decontaminated before it can leave the facility.[10]
Biosafety level 4 laboratories are used for diagnostic work and research on easily transmitted pathogens which can cause fatal disease. These include a number of viruses known to cause viral hemorrhagic fever such as Marburg virus, Ebola virus, Lassa virus, and Crimean-Congo hemorrhagic fever. Other pathogens handled at BSL-4 include Hendra virus, Nipah virus, and some flaviviruses. Additionally, poorly characterized pathogens which appear closely related to dangerous pathogens are often handled at this level until sufficient data are obtained either to confirm continued work at this level, or to permit working with them at a lower level.[14] This level is also used for work with Variola virus, the causative agent of smallpox, though this work is only performed at the Centers for Disease Control and Prevention in Atlanta, United States, and the State Research Center of Virology and Biotechnology in Koltsovo, Russia.[17]
 Regular inspection of positive-pressure suits to locate any leaks[18]
Regular inspection of positive-pressure suits to locate any leaks[18]
 The circular containment tube separates the patient table in the "hot" zone (pathogen present) from the "cold" zone around this MRI machine.
The circular containment tube separates the patient table in the "hot" zone (pathogen present) from the "cold" zone around this MRI machine._door.jpg) Air pressure resistant (APR) door to separate the hot and cold zones
Air pressure resistant (APR) door to separate the hot and cold zones Working inside a BSL-4 lab with air hoses providing positive air pressure.
Working inside a BSL-4 lab with air hoses providing positive air pressure. Inside a Class III biological safety cabinet with an aerosol control platform
Inside a Class III biological safety cabinet with an aerosol control platform Effluent decontamination system of a BSL-4 lab of NIAID
Effluent decontamination system of a BSL-4 lab of NIAID
BSL-4 facilities for extraterrestrial samples
Sample-return missions that bring back to Earth samples obtained from a Category V body must be curated at facilities rated BSL-4. Because the existing BSL-4 facilities in the world do not have the complex requirements to ensure the preservation and protection of Earth and the sample simultaneously,[19] there are currently at least two proposals to build a BSL-4 facility dedicated to curation of restricted (potentially biohazardous) extraterrestrial materials.
The first is the European Sample Curation Facility (ESCF),[20][21] proposed to be built in Vienna, which would curate non-restricted samples as well as perform BSL-4 processing of restricted material obtained from Category V bodies such as Mars, Europa, and Enceladus.[20] The other proposal is by NASA and is tentatively known as the Mars Sample-Return Receiving facility (MSRRF).[22] At least three different designs were submitted in 2009.[19] If funded, this American facility would be expected to take 7 to 10 years from design to completion,[23][24] and an additional two years is recommended for the staff to become proficient and accustomed to the facilities.[23] NASA is also assessing a 2017 proposal to build a mobile and modular BSL-4 facility to secure a sample return capsule at the landing site to conduct preliminary biohazard analyses.[25] After completion of biohazard testing, decisions could be made to sterilize the sample or transport all or portions to a permanent quarantine storage facility anywhere in the world.[25]
The systems of such facilities must be able to contain unknown biohazards, as the sizes of any putative alien microorganisms are unknown. Ideally, it should filter particles down to 10 nanometers, and release of a particle 50 nanometers or larger is unacceptable under any circumstance.[26]
List of BSL-4 facilities
According to a U.S. Government Accountability Office (GAO) report published on 4 October 2007, a total of 1,356 CDC/USDA registered BSL-3 facilities were identified throughout the United States.[27] Approximately 36% of these laboratories are located in academia. 15 BSL-4 facilities were identified in the U.S. in 2007, including nine at federal labs.[27]
The following is a list of existing BSL-4 facilities worldwide.
| Name | Location | Country | Date established |
Description |
|---|---|---|---|---|
| National Service of Healthcare and Agriculture Quality (SENASA) | Buenos Aires | Argentina | Diagnostic laboratory for Foot-and-mouth disease.[28] | |
| Australian Centre for Disease Preparedness | Geelong, Victoria | Australia | 1985 | Capable of housing from large experimental animals to insects under conditions that exceed all BSL 4 requirements. The antecedent of all such facilities developed since the 1980s. Arguably the most researched design and construction project ever. AAHL is subdivided into a number of isolation zones that can be managed at differing containment levels concurrently. CSIRO AAHL Project Manager and Architect, William Curnow, provided technical reviews to Canadian, Indian, UK and French Authorities and consulted with Dr Jerry Callis [PIADC] to UN FAO on matters of bio-containment. Formerly known as the Australian Animal Health Laboratory (AAHL) and renamed to Australian Centre for Disease Preparedness April 2020 |
| University of Melbourne – Doherty Institute for Infection and Immunity | Melbourne, Victoria | Australia | 2014 | Diagnostic reference lab.[29][30] |
| National High Security Laboratory | Melbourne, Victoria | Australia | Operates under the auspice of the Victoria Infectious Diseases Reference Laboratory.[31] | |
| Laboratório Nacional Agropecuário de Minas Gerais (Lanagro/MG) | Pedro Leopoldo, Minas Gerais | Brazil | 2014 | Focus on Agropecuary diseases and diagnostics.[32] |
| National Microbiology Laboratory | Winnipeg, Manitoba | Canada | 1999[33] | Located at the Canadian Science Centre for Human and Animal Health, it is jointly operated by the Public Health Agency of Canada and the Canadian Food Inspection Agency.[34] |
| Wuhan Institute of Virology of the Chinese Academy of Sciences | Wuhan, Hubei | China | 2015 | Wuhan Institute of Virology has existed since 1956 and already hosted BSL3 laboratories. A BSL4 facility was completed in 2015, and became the first BSL-4 laboratory in China.[35] |
| Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences | Harbin, Heilongjiang | China | 2018 | Harbin Veterinary Research Institute researches prevention and control of major infectious diseases. China's second, and the first for large animals, BSL-4 lab.[36] |
| Biological Defense Center | Těchonín, Pardubice Region | Czech Republic | 1971, rebuilt 2003–2007 | Hospital and research facility. Located at the Centrum biologické ochrany (Biological Defense Center). Operated by Army of the Czech Republic.[37] |
| French Armed Biomedical Research Institute, French Defence Health Service | Brétigny-sur-Orge, Essonne | France | French Army laboratory.[38] | |
| Jean Mérieux BSL-4 Laboratory | Lyon, Metropolis of Lyon | France | 1999 | Built and owned by the Fondation Mérieux. Since 2004, operated by INSERM.[39] |
| Laboratoire de la DGA | Vert-le-Petit, Essonne | France | 2013 | Operated by the Ministry of Defense.[40] |
| Centre International de Recherches Médicales de Franceville | Franceville, Haut-Ogooué Province | Gabon | This facility is operated by a research organization supported by both Gabonese (mainly) and French governments, and is West Africa's only P4 lab (BSL-4).[41] | |
| Robert Koch Institute | Berlin | Germany | 2015 | Diagnostic and experimental lab facility.[42] |
| Bernhard Nocht Institute for Tropical Medicine | Hamburg | Germany | 2014 | Part of the Leibniz Center Infection. National reference lab for tropical viruses.[43] |
| Friedrich Loeffler Institute | Isle of Riems, Greifswald, Mecklenburg-Vorpommern | Germany | 2010 | Focus on animal viral diseases and diagnostics.[44] |
| Philipps University of Marburg | Marburg, Hesse | Germany | 2008 | Focuses on hemorrhagic fever viruses.[45] |
| National Center for Epidemiology | Budapest | Hungary | 1998 | Division of Virology operates three WHO National Reference Laboratories. The BSL-4 biosafety laboratory provides a modern means to process dangerous imported zoonotic viral pathogens.[46] |
| University of Pécs | Pécs | Hungary | 2016 | Opened in 2016, part of "Szentágothai János Kutatóközpont". |
| High Security Animal Disease Laboratory (HSADL) | Bhopal, Madhya Pradesh | India | 1998 | This facility deals especially to zoonotic organisms and emerging infectious disease threats.[47] |
| Centre for Cellular and Molecular Biology | Hyderabad, Telangana | India | 2009 | National BSL-4 Containment Facility for Human Infectious Diseases.[48] |
| National Institute of Virology | Pune, Maharashtra | India | 2012 | India's first BSL4 lab and the most advanced.[49] |
| Istituto Nazionale per le Malattie Infettive | Rome, Lazio | Italy | 1997 | The "National Institute of Infectious Diseases" used to operate within the Lazzaro Spallanzani hospital; the facility is now independent and is home to five BSL-3 labs as well as a single BSL-4 laboratory, which was completed in 1997.[50] |
| National Institute for Infectious Diseases | Musashimurayama, Tokyo | Japan | 2015 | Located at National Institute for Infectious Diseases, Department of Virology I. Built in 1981; operated at BSL-3 until 2015 due to opposition from nearby residents.[51] |
| Institute of Physical and Chemical Research (RIKEN) | Tsukuba, Ibaraki Prefecture | Japan | 1984 | Facility completed in 1984 but not operated as BSL-4 due to local opposition.[52] |
| State Research Center of Virology and Biotechnology (VECTOR) | Koltsovo, Novosibirsk Oblast | Russia | One of two WHO-approved facilities for work on variola virus.[17] | |
| National Institute for Communicable Diseases | Johannesburg, Gauteng | South Africa | 2002 | [53] |
| Korea Centers for Disease Control and Prevention | Cheongju, North Chungcheong Province | South Korea | 2017 | First BSL-4 Lab in South Korea |
| Public Health Agency of Sweden | Solna, Stockholm County | Sweden | 2001 | The only BSL-4 facility in the Nordic region. Constructed for research and diagnostics of hemorrhagic fever viruses.[54] |
| University Hospital of Geneva | Geneva, Canton of Geneva | Switzerland | "Glove box" type laboratory; primarily for handling clinical samples.[55] | |
| Spiez Laboratory | Spiez, Canton of Bern | Switzerland | 2013 | Run by Federal Office for Civil Protection and the Federal Department of Defence, Civil Protection and Sports.[56] |
| The Institute of Virology and Immunology IVI[57] | Mittelhäusern, Canton of Bern | Switzerland | Part of the Food Safety and Veterinary Office (FSVO).[58] Primary purpose is diagnostics of highly pathogenic viruses.[56] | |
| Institute of Preventive Medicine | National Defense University | Taiwan | 1983 | [59] |
| Francis Crick Institute | Camden, Greater London | United Kingdom | 2015 | Has BSL-4 space but does not work on human pathogens.[60] |
| Public Health England's Centre for Infections | Colindale, Greater London | United Kingdom | Department of Health laboratory. Diagnostics for various viral diseases.[61] Part of the European Network of Biosafety-Level-4 Laboratories.[62] | |
| National Institute for Medical Research | Mill Hill, Greater London | United Kingdom | Medical Research Council laboratory. Research and diagnostics for highly pathogenic viruses. Closed in 2017 and work moved to the Francis Crick Institute. Site demolished in 2018. [61] | |
| National Institute for Biological Standards and Control | Potters Bar, Hertfordshire | United Kingdom | Department of Health and Home Office laboratory. Develop assays and reagents for research on virulent pathogens.[61] | |
| Animal and Plant Health Agency | Addlestone, Surrey | United Kingdom | Department for Environment, Food and Rural Affairs laboratory. Diagnostics and research for animal diseases.[61] | |
| Institute for Animal Health | Pirbright, Surrey | United Kingdom | Biotechnology and Biological Sciences Research Council laboratory. Research on highly pathogenic animal diseases.[61] | |
| Merial Animal Health | Pirbright, Surrey | United Kingdom | Private lab. Produces vaccines against foot and mouth disease and bluetongue disease.[61] | |
| Centre for Emergency Preparedness and Response | Porton Down, Wiltshire | United Kingdom | Department of Health laboratory. Diagnostics and research for haemorrhagic fever viruses.[61] Part of the European Network of Biosafety-Level-4 Laboratories.[62] | |
| Defence Science and Technology Laboratory | Porton Down, Wiltshire | United Kingdom | Ministry of Defence laboratory. Focuses on protection from biological weapons.[61] | |
| Centers for Disease Control and Prevention, Division of Vector Borne Diseases | Fort Collins, Colorado | United States | A BSL 3/4 facility that operates in connection with some of Colorado State University's biomedical research programs. The location specializes in arboviral and bacterial diseases.[63] | |
| Centers for Disease Control and Prevention | Atlanta, Georgia | United States | Currently operates in two buildings. One of two facilities in the world that officially hold smallpox.[17] | |
| Georgia State University | Atlanta, Georgia | United States | 1997 | Research focus on B virus.[64] |
| National Bio and Agro-Defense Facility (NBAF), Kansas State University | Manhattan, Kansas | United States | 2022 (expected) | Under construction. Facility to be operated by the Department of Homeland Security, and replace the Plum Island Animal Disease Center. Expected to be operational by 2022–2023.[65] |
| National Institutes of Health (NIH) | Bethesda, Maryland | United States | Located on the NIH Campus, it currently only operates with BSL-3 agents.[66] | |
| Integrated Research Facility | Fort Detrick, Maryland | United States | Operated by National Institute of Allergy and Infectious Diseases (NIAID). Focuses on animal models of human diseases.[67] | |
| National Biodefense Analysis and Countermeasures Center | Fort Detrick, Maryland | United States | Operated by the Department of Homeland Security. Focus on potential bioterrorism threats.[68] | |
| US Army Medical Research Institute of Infectious Diseases (USAMRIID) | Fort Detrick, Maryland | United States | 1969 | Run by the U.S. Army. Research focuses on biological threats to the U.S. military.[69][70] |
| National Emerging Infectious Diseases Laboratory (NEIDL), Boston University | Boston, Massachusetts | United States | Built 2008, Opened 2012[71], BSL-4 Approval in 2017 [72] | Focus on potential threats to public health.[73] |
| Rocky Mountain Laboratories Integrated Research Facility | Hamilton, Montana | United States | 2008 | NIAID laboratory. Focus on vector-borne diseases.[74] |
| Galveston National Laboratory, National Biocontainment Facility | Galveston, Texas | United States | Opened in 2008, facility is operated by the University of Texas Medical Branch.[75] | |
| Shope Laboratory | Galveston, Texas | United States | 2004 | Operated by the University of Texas Medical Branch.[76] |
| Texas Biomedical Research Institute | San Antonio, Texas | United States | 1999 | The only privately owned BSL-4 lab in the US.[77] |
Safety concerns
A North Carolina Mosquito & Vector Control Association (NCMVCA) study highlighted safety concerns. In the United States, laboratories can be funded by federal, state, private, non-profit, or academically. The last accounts for 72% of the funding. There is no central monitoring agency accountable for monitoring laboratories and standards vary according to funding, the age of the laboratory, and is dependent on the size and whether it is SA approved.[78]
High-containment labs that are registered with the Centers for Disease Control and Prevention (CDC) and the U.S. Department of Agriculture's (USDA) Select Agent Program must adhere to Department of Defense standards.[79] No single federal agency, according to 12 agencies' responses to a GSA survey, has the mission to track the overall number of BSL-3 and BSL-4 labs in the United States. This means no agency is responsible for determining the risks associated with the proliferation of these labs.[80]
See also
References
- "Integrated Research Facility". niaid.nih.gov. NIAID. Archived from the original on 28 November 2014. Retrieved 14 November 2014.
- Chosewood LC, Wilson DE, eds. (2009). Biosafety in Microbiological and Biomedical Laboratories (5th ed.). Centers for Disease Control and Prevention. ISBN 0-1608-5042-8. Retrieved 1 April 2020.
- Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work (seventh individual directive within the meaning of Article 16(1) of Directive 89/391/EEC)
- Canada, Public Health Agency of. "Chapter 2: The Laboratory Biosafety Guidelines: 3rd Edition 2004 – Biological safety - Canada.ca". www.canada.ca. Archived from the original on 23 February 2018. Retrieved 7 May 2018.
- Laboratory Safety Monograph: A Supplement to the NIH Guidelines for Recombinant DNA Research. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health, National Cancer Institute, Office of Research Safety. 1978. passim.
- Covt, Norman M. (1997), “A History of Fort Detrick, Maryland” Archived 2008-09-22 at the Wayback Machine, 3rd edition. Kaempf retired from Fort Detrick in 1994, having completed more than 50 years service. He was chief of the mechanical branch, Directorate of Engineering and Housing.
- Manuel S. Barbeito; Richard H. Kruse. "A History of the American Biological Safety Association". American Biological Safety Association. Archived from the original on 20 June 2008. Retrieved 14 August 2008.
- "American Biological Safety Association Collection : NAL Collections : National Agricultural Library". United States Department of Agriculture: National Agricultural Library. 11 February 2009. Archived from the original on 27 February 2009. Retrieved 11 February 2009.
- "CSIRO: Geelong - Australian Animal Health Laboratory".
- "Section IV-Laboratory Biosafety Level Criteria". Biosafety in Microbiological and Biomedical Laboratories, 5th ed (PDF). U.S. Department of Health and Human Services. December 2009. pp. 30–59. Archived (PDF) from the original on 9 April 2016. Retrieved 2 April 2016.
- Richmond JY. "The 1, 2, 3's of Biosafety Levels" (PDF). Archived (PDF) from the original on 19 March 2015. Retrieved 2 April 2016.
- "Health & Safety Manual - Biological Safety". Columbia University Environmental Health and Safety. Archived from the original on 27 March 2016. Retrieved 2 April 2016.
- "Section III-Principles of Biosafety". Biosafety in Microbiological and Biomedical Laboratories, 5th ed (PDF). U.S. Department of Health and Human Services. December 2009. pp. 22–28. Archived (PDF) from the original on 10 March 2016. Retrieved 9 April 2016.
- For a list of infectious agents and the recommended biosafety level at which they should be studied, see "Section VIII-Agent Summary Statements". Biosafety in Microbiological and Biomedical Laboratories, 5th ed. U.S. Department of Health and Human Services. December 2009. pp. 123–289. Archived (PDF) from the original on 27 March 2016. Retrieved 9 April 2016.
- "Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19)". Coronavirus Disease 2019 (COVID-19) Lab Biosafety Guidelines. Centers for Disease Control and Prevention. Retrieved 1 April 2020.
- "China CDC remobilizes P3 lab to facilitate COVID-19 nucleic acid testing in Beijing". Global Times. 17 June 2020.
- "Section VIII-Agent Summary Statements". Biosafety in Microbiological and Biomedical Laboratories, 5th ed (PDF). U.S. Department of Health and Human Services. December 2009. p. 219. Archived (PDF) from the original on 13 May 2016. Retrieved 4 May 2016.
- Seligson, Susan (7 March 2013). "Video Offers Glimpse of Biosafety Level 4 Lab Science webcast "threads the NEIDL"". BU Today. Archived from the original on 10 December 2014. Retrieved 5 December 2014.
- How to Protect Mars Samples on Earth. Jeremy Hsu, Space.com. 3 December 2009.
- EURO EURO-CARES Extraterrestrial Sample Curation Facility: Architecture as an enabler of science. (PDF) Aurore Hutzler, Emre Kilic, Allan Bennett, Ludovic Ferrière. 47th International Conference on Environmental Systems, 16-20 July 2017, Charleston, South Carolina. Document ICES-2017-323.
- EURO-CARES. European Curation of Astromaterials Returned from Exploration of Space. Accessed: 25 September 2018.
- Ronald Atlas (2002). "Mars Sample Return Receiving Facility" (PDF). NASA.
- "7: "Sample-Receiving Facility and Program Oversight"". Assessment of Planetary Protection Requirements for Mars Sample Return Missions (Report). National Research Council. 2009. p. 59.
- Mars Sample Return: Issues and Recommendations (Planetary Protection Office Summary) Task Group on Issues in Sample Return. National Academies Press, Washington, DC (1997)
- Mobile/Modular BSL-4 Facilities for Meeting Restricted Earth Return Containment Requirements. M. J. Calaway, F. M. McCubbin, J. H. Allton, R. A. Zeigler, and L. F. Pace. (PDF) NASA. 2017.
- European Science Foundation - Mars Sample Return backward contamination - Strategic advice and requirements Archived 2016-06-02 at the Wayback Machine
- "High-Containment Biosafety Laboratories: Preliminary Observations on the Oversight of the Proliferation of BSL-3 and BSL-4 Laboratories in the United States" (PDF). United States Government Accountability Office. 4 October 2007. Archived (PDF) from the original on 12 February 2016. Retrieved 26 May 2016.
- "Risk Analysis:Risk of Importing Foot-and-Mouth Disease in Susceptible Species and Products from a region of Patagonia, Argentina" (PDF). U.S. Department of Agriculture, National Import Export Services, Veterinary Services. January 2014. pp. 60–62. Archived (PDF) from the original on 21 October 2016. Retrieved 3 April 2016.
- "Members: The Doherty Institute for Infection and Immunity". Global Virus Network. Archived from the original on 20 March 2016. Retrieved 3 April 2016.
- Racaniello V (14 July 2014). "Visiting biosafety level-4 laboratories". Virology Blog. Archived from the original on 18 April 2016. Retrieved 3 April 2016.
- "Laboratories: High Security/Quarantine". Victorian Infectious Diseases Reference Laboratory. Archived from the original on 19 April 2016. Retrieved 8 April 2016.
- "Lanagro/MG é o primeiro do Brasil com nível de biossegurança máximo". MAPA - Ministério da Agricultura, Pecuária e Abastecimento. August 2014. Archived from the original on 23 February 2018. Retrieved 22 February 2018.
- http://webarchive.bac-lac.gc.ca:8080/wayback/20071213074113/http://www.hc-sc.gc.ca/ahc-asc/media/nr-cp/1999/1999_81_e.html
- "National Microbiology Laboratory (NML) Overview". Public Health Agency of Canada. Archived from the original on 21 March 2016. Retrieved 8 April 2016.
- "China Inaugurates the First Biocontainment Level 4 Laboratory in Wuhan". Wuhan Institute of Virology, Chinese Academy of Sciences. 3 February 2015. Archived from the original on 3 March 2016. Retrieved 9 April 2016.
- "China launches high-level biosafety lab". Xinhua. 8 August 2018. Archived from the original on 14 October 2018. Retrieved 13 March 2019.
- "Biological Defence Department at Techonin". Ministry of Defense & Armed Forces of the Czech Republic. Archived from the original on 26 April 2016. Retrieved 9 April 2016.
- "Un laboratoire militaire hautement sécurisé à Brétigny en 2015". Le Parisien (in French). 20 May 2014. Retrieved 5 March 2020.
- "Jean Mérieux BSL-4 Laboratory". Fondation Mérieux. Archived from the original on 6 May 2016. Retrieved 11 April 2016.
- "Inauguration du laboratoire biologique P4 de la DGA" (in French). Ministére de la Défense. Archived from the original on 8 May 2016. Retrieved 11 April 2016.
- "Centre International de Recherches Medicales de Franceville" (in French). CIRMF. Archived from the original on 15 October 2014. Retrieved 30 September 2014.
- "Das Hochsicherheitslabor im Robert Koch-Institut". Robert Koch Institut. Archived from the original on 19 May 2016. Retrieved 16 April 2016.
- "Bernhard Nocht Institute for Tropical Medicine (BNI)". Heinrich Pette Institute. Archived from the original on 27 April 2016. Retrieved 16 April 2016.
- "Friedrich Loeffler Institute, Germany". Caverion. Archived from the original on 22 April 2016. Retrieved 16 April 2016.
- "Philipps-University Marburg". Philipps-University Marburg. Archived from the original on 11 June 2016. Retrieved 16 April 2016.
- "Division of Virology". Országos Epidemiológiai Központ. Archived from the original on 24 September 2013. Retrieved 16 April 2016.
- "Bio-containment Laboratory". National Institute of High Security Animal Diseases, India. Archived from the original on 19 March 2016. Retrieved 20 April 2016.
- "Stone laid for stem cell research lab in Hyderabad". The Hindu. Archived from the original on 16 January 2016. Retrieved 25 April 2016.
- "NIV Prune lab gets BSL-4". The Hindu. Archived from the original on 16 August 2017. Retrieved 24 April 2016.
- "Storia dell'Istituto" (in Italian). IRCCS Lazzaro Spallanzani. Archived from the original on 7 March 2014. Retrieved 1 May 2016.
- "Deadly disease lab opens amid local fears". Japan Times. 15 October 2015. Archived from the original on 28 April 2016. Retrieved 1 May 2016.
- "Bio lab handling highly dangerous agents to open in suburban Tokyo". Japan Bullet. 3 August 2015. Archived from the original on 15 March 2018. Retrieved 14 March 2018.
- "South Africa National Institute for Communicable Diseases". African National Public Health Institutes. Archived from the original on 18 March 2016. Retrieved 4 May 2016.
- "P4-laboratoriet vid Folkhälsomyndigheten" (in Swedish). Public Health Agency of Sweden. Archived from the original on 15 October 2014. Retrieved 8 October 2014.
- Cherpillod, P. "Management of suspect viral hemorrhagic fever patient in Geneva". Schweizerische Union fur Labormedizin. Archived from the original on 16 August 2016. Retrieved 10 May 2016.
- "Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on their Destruction" (PDF). Switzerland Federal Department of Defence, Civil Protection, and Sports. 2016. Archived (PDF) from the original on 3 June 2016. Retrieved 10 May 2016.
- https://www.ivi.admin.ch/ivi/en/home.html
- https://www.blv.admin.ch/blv/en/home.html
- "Case of SARS reported in a laboratory research worker in Taiwan". Eurosurveillance. 18 December 2003. Archived from the original on 11 June 2016. Retrieved 18 May 2016.
- Ewen Callaway (6 June 2013). "London biomedical hub sets its research agenda". Nature. Archived from the original on 7 June 2016. Retrieved 26 May 2016.
- Davison N; Lentzos F (2012). "E8: High-Containment Laboratories-UK Case Study". Biosecurity Challenges of the Global Expansion of High-Containment Biological Laboratories. pp. 176–177. ISBN 978-0-309-22575-5. Archived from the original on 23 June 2016. Retrieved 26 May 2016.
- Nisii, Carla; Castilletti, Concetta; Raoul, Hervé; Hewson, Roger; Brown, David; Gopal, Robin; Eickmann, Markus; Gunther, Stephan; Mirazimi, Ali; Koivula, Tuija; Feldmann, Heinz; Di Caro, Antonino; Capobianchi, Maria R.; Ippolito, Giuseppe (2013). "Biosafety Level-4 Laboratories in Europe: Opportunities for Public Health, Diagnostics, and Research". PLOS Pathogens. 9 (1): e1003105. doi:10.1371/journal.ppat.1003105. PMC 3547859. PMID 23349630.
- "About DVBD - Division of Vector-Borne Diseases (DVBD) - NCEZID - CDC". www.cdc.gov. 17 April 2018. Archived from the original on 16 December 2017. Retrieved 7 May 2018.
- "Operating a BSL-4 Laboratory in a University Setting". Tradeline. 16 December 2003. Archived from the original on 30 June 2016. Retrieved 28 May 2016.
- "Leveraging the National Bio and Agro-defense Facility". Kansas State University. Archived from the original on 10 June 2016. Retrieved 28 May 2016.
- "An Integrated Research Facility: Questions and Answers". National Institute of Allergy and Infectious Diseases. Archived from the original on 22 June 2016. Retrieved 28 May 2016.
- "Integrated Research Facility Overview". National Institute of Allergy and Infectious Disease. Archived from the original on 5 July 2016. Retrieved 28 May 2016.
- "National Biodefense Analysis and Countermeasures Center". Department of Homeland Security. Archived from the original on 20 May 2016. Retrieved 28 May 2016.
- "USAMRIID: Biodefense Solutions to Protect our Nation". U.S. Army Medical Department. Archived from the original on 5 June 2016. Retrieved 28 May 2016.
- "USAMRIID Biological Safety". U.S. Army Medical Department. Archived from the original on 18 May 2016. Retrieved 28 May 2016.
- "NEIDL Goes Public: BU Biosafety Labs Offer Tours to Press, Politicians". Retrieved 30 December 2018.
- "NEIDL BSL-4 Lab Gets Green Light". Retrieved 30 December 2018.
- "National Emerging Infectious Diseases Laboratories: About - Mission and Safety". Boston University. Archived from the original on 4 June 2016. Retrieved 28 May 2016.
- "Rocky Mountain Labs Overview". National Institute for Allergy and Infectious Disease. Archived from the original on 29 April 2016. Retrieved 28 May 2016.
- "Galveston National Laboratory Fact Sheet". Archived from the original on 5 October 2014. Retrieved 30 September 2014.
- "Center for Biodefense and Emerging Infectious Diseases: Safety and Biocontainment". UTMB Health. Archived from the original on 5 January 2016. Retrieved 28 May 2016.
- "About Texas Biomed: Biosafety Level 4 Laboratory". Texas Biomedical Research Institute. Archived from the original on 3 April 2016. Retrieved 3 April 2016.
- NCMVCA study Archived 2017-01-31 at the Wayback Machine- Retrieved 2017-01-19
- DoD Safety Standards for Microbiological and Biomedical Laboratories Archived 2017-01-25 at the Wayback Machine- Retrieved 2017-01-19
- GAO publication Archived 2017-01-20 at the Wayback Machine- Retrieved 2017-01-19