Ixabepilone
Ixabepilone (INN; also known as azaepothilone B, codenamed BMS-247550) is a pharmaceutical drug developed by Bristol-Myers Squibb as a chemotherapeutic medication for cancer.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Ixempra |
| Other names | Azaepothilone B |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608042 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous infusion |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | 67 to 77% |
| Metabolism | Extensive, hepatic, CYP3A4-mediated |
| Elimination half-life | 52 hours |
| Excretion | Fecal (mostly) and renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.736 |
| Chemical and physical data | |
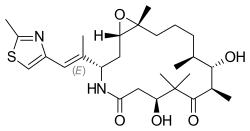
| Formula | C27H42N2O5S |
| Molar mass | 506.70 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
History
Ixabepilone is a semi-synthetic analog of epothilone B, a natural chemical compound produced by Sorangium cellulosum.[2] Epothilone B itself could not be developed as a pharmaceutical drug because of poor metabolic stability and pharmacokinetics.[3] Ixabepilone was designed through medicinal chemistry to improve upon these properties.[3]
Pharmacology
Much like Taxol, Ixabepilone acts to stabilize microtubules.[4][5][6] It is highly potent, capable of damaging cancer cells in very low concentrations, and retains activity in cases where tumor cells are insensitive to taxane type drugs.[7]
Approval
On October 16, 2007, the U.S. Food and Drug Administration approved ixabepilone for the treatment of aggressive metastatic or locally advanced breast cancer no longer responding to currently available chemotherapies.[8] In November 2008, the EMEA has refused a marketing authorisation for Ixabepilone.[9]
Ixabepilone is administered through injection, and is marketed under the trade name Ixempra.
Clinical uses
Ixabepilone, in combination with capecitabine, has demonstrated effectiveness in the treatment of metastatic or locally advanced breast cancer in patients after failure of an anthracycline and a taxane.[10]
It has been investigated for use in treatment of non-Hodgkin's lymphoma.[11] In pancreatic cancer phase two trial it showed some promising results (used alone). Combination therapy trials are ongoing.[7]
References
- "More information on cancer drugs". www.cancer.org.
- Goodin S (May 2008). "Novel cytotoxic agents: epothilones". Am J Health Syst Pharm. 65 (10 Suppl 3): S10–5. doi:10.2146/ajhp080089. PMID 18463327.
- Lee FY, Borzilleri R, Fairchild CR, et al. (December 2008). "Preclinical discovery of ixabepilone, a highly active antineoplastic agent". Cancer Chemother. Pharmacol. 63 (1): 157–66. doi:10.1007/s00280-008-0724-8. PMID 18347795.
- Lopus, M; Smiyun, G; Miller, H; Oroudjev, E; Wilson, L; Jordan, MA (2015). "Mechanism of action of ixabepilone and its interactions with the βIII-tubulin isotype". Cancer Chemother Pharmacol. 76 (5): 1013–24. doi:10.1007/s00280-015-2863-z. PMID 26416565.
- Denduluri N, Swain SM (March 2008). "Ixabepilone for the treatment of solid tumors: a review of clinical data". Expert Opin Investig Drugs. 17 (3): 423–35. doi:10.1517/13543784.17.3.423. PMID 18321240.
- Goodin S (November 2008). "Ixabepilone: a novel microtubule-stabilizing agent for the treatment of metastatic breast cancer". Am J Health Syst Pharm. 65 (21): 2017–26. doi:10.2146/ajhp070628. PMID 18945860.
- M. Vulfovich; Rocha-Lima, C; et al. (2008). "Novel advances in pancreatic cancer treatment". Expert Rev Anticancer Ther. 8 (6): 993–1002. doi:10.1586/14737140.8.6.993. PMID 18533808.
- "FDA Approves IXEMPRA(TM) (ixabepilone), A Semi-Synthetic Analog Of Epothilone B, For The Treatment Of Advanced Breast Cancer". Medical News Today.
- London, 20 November 2008 Doc. Ref. EMEA/602569/2008
- Thomas ES, Gomez HL, Li RK, et al. (November 2007). "Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment". J. Clin. Oncol. 25 (33): 5210–7. doi:10.1200/JCO.2007.12.6557. PMID 17968020. Archived from the original on 2013-04-15.
- Aghajanian C, Burris HA, Jones S, et al. (March 2007). "Phase I study of the novel epothilone analog ixabepilone (BMS-247550) in patients with advanced solid tumors and lymphomas". J. Clin. Oncol. 25 (9): 1082–8. doi:10.1200/JCO.2006.08.7304. PMID 17261851. Archived from the original on 2013-04-15.