BEND2 (protein)
BEND2 is a protein that in humans is encoded by the BEND2 gene.[1] It is also found in other vertebrates, including mammals, birds, and reptiles.[1] The expression of BEND2 in Homo sapiens is regulated and occurs at high levels in the skeletal muscle tissue of the male testis and in the bone marrow.[2][3][4] The presence of the BEN domains in the BEND2 protein indicates that this protein may be involved in chromatin modification and regulation.[5]
| BEND2 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
Gene
Common aliases
BEND2 stands for BEN domain containing 2 and is also known as CXorf20 (HGNC ID: 28509).[1][6][7]
Locus and size
The locus for BEND2 is on the minus strand of the X chromosome at Xp22.13. The gene is approximately 58 kilobases in length.[1]
mRNA
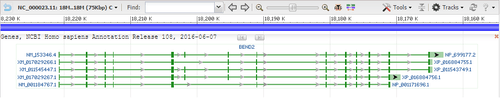
Alternative splicing
BEND2 contains 14 exons which undergo alternative splicing to create five transcript variants that vary from 4,720 base pairs (bp) to 2,144 bp in the mature mRNA.[1][7][3] The longest and most complete transcript of the gene, variant 1, encodes isoform 1 of the BEND2 protein (NP_699177.2).[1]
5' and 3'UTR
The untranslated regions (UTR) flanking the coding sequence of BEND2 at the 5' and 3' end of the mature mRNA molecule contain sites for RNA-binding proteins, including RBMX, pum2, and EIF4B as well as microRNA binding sites. The 5'UTR also contains an upstream in-frame stop codon and the 3'UTR contains a polyadenylation signal sequence.
Protein (Isoform 1)
Molecular weight and internal composition
The predicted molecular weight is 87.9 kDal.[10][11]
The predicted isoelectric point is pH 5.07.[12]
The internal composition is enriched for serine residues.[10]
Isoforms
Corresponding to the five alternative transcripts of BEND2, the protein encoded by this gene is found in two isoforms (1 and 2) as well as three predicted structures (X1, X2, and X3). These isoforms range from 813 to 645 amino acids in length.[1] Isoform 1 is 799 amino acids in length.[13]
Subcellular location
The presence of nuclear localization signals within the amino acid sequence or primary structure of the BEND2 protein leads to a prediction of subcellular localization in the nucleus.[14] The pat7 [(P-X(1-3)-(3-4K/R)] signal and a nuclear bipartite signal are both found near the N-terminus of the protein.[14][15]
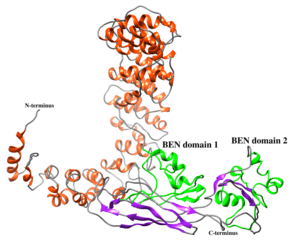
Structure
The secondary structure for BEND2 is unclear, in particular at the N-terminus, which is poorly conserved between orthologs. The C-terminus contains two BEN domains, which are predicted to form a series of alpha helices.[1][5]
Post-translational modifications
Based on its primary structure, BEND2 is predicted to undergo N-terminus acetylation, glycation of several lysine residues, SUMOlation, a SUMO interaction at the N-terminus, S-palmitoylation, and extensive phosphorylation.[16]
Interacting Proteins
BEND2 is found to interact with the following proteins through experimental yeast two-hybrid screens or pull down assays.
| Experiment type | Protein | Protein Function | Associated diseases |
|---|---|---|---|
| Two-hybrid screen | Ataxin 1(ATXN1)[17] | Chromatin-binding factor; RNA metabolism | Spinocerebellar ataxia 1/spinocerebellar degeneration |
| Two-hybrid screen | Splicing factor 3A subunit 2(SF3A2)[18] | Activation of U2 snRNP; microtubule-binding protein | |
| Two-hybrid screen | LIM Homeobox 2 (LHX2)[18] | Transcriptional regulator for cell differentiation; sequence-specific DNA binding | Schizencephaly |
| Two-hybrid screen | Proline Rich 20D (PRR20D)[18] | Unknown function | |
| Pull down assay | Amyolid precursor protein (APP)[19] | Cell surface receptor in neurons; cleaved to form transcriptional activators | Cerebral amyloid angiopathy; Alzheimer's disease |
BEN Domains (protein feature)
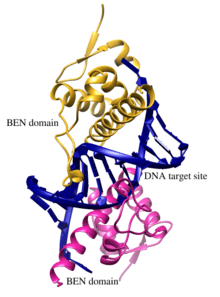
BEND2 has two BEN domains at its C-terminus.[1] BEN domains are found in a diverse array of proteins and are predicted to be important for chromatin remodeling as well as for the recruitment of chromatin-modifying factors utilized during the process of transcriptional regulation of gene expression.[5] BEN domains are predicted to form four alpha helices that allow this domain to interact with its DNA target.[5][20]
Dai et al. 2013 showed that the Drosophila melanogaster Insensitive (Insv) gene and corresponding protein has no domains of known chemical function yet it contains a single BEN domain. They illustrated the activity of the Insv protein in transcriptional regulation of genes and obtained a crystal structure of two Insv BEN domains interacting with their DNA target site.[20]
Expression
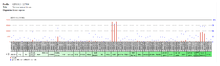
Tissue expression pattern
The expression of the BEND2 gene is regulated and it is therefore not ubiquitously expressed in the human body. High expression occurs in the testis and in the bone marrow.[21] The NCBI EST profile for this gene shows expression only in the testis and in the muscle.[22]
Transcriptional regulation of expression
The promoter regulating expression of BEND2 (GXP_2567556) is 1255 base pairs in length and is located directly upstream of the BEND2 gene. It regulates transcription of all five transcriptional variants of BEND2.[23] Genomatix's MatInspector program predicted 418 transcription factor binding sites within the BEND2 promoter, including for SRY, neurogenin, interferon regulatory factor-3 (IRF-3), Ikaros2, and TCF/LEF-1.
Homology
Paralogs
The BEND2 protein has no known paralogs within the human genome.[24]
BEN-domain containing gene family
The BEND2 gene belongs to a family of human genes known as "BEN-domain containing”. This includes BANP (BEND1), BEND3, BEND4, BEND5, BEND6, BEND7, NACC1 (BEND8), and NACC2 (BEND9). The loci for these genes are spread throughout the human genome.[25] Each of these genes contains between one and four BEN domains. Except for at these motifs, the genes of the BEN family do not have similar sequences.
Orthologs
The BEND2 gene is conserved across evolutionary time as it has 114 known orthologs in a wide range of vertebrate species including mammals, birds, crocodilia, and amphibians.[26] The BEND2 protein has 42 known orthologs.[27] The C-terminus of the protein, the location of its BEN domains, is highly conserved; however, the N-terminus is not well conserved, even within the order of Primates.
| Genus/species | Common name | Order | Date of divergence from H. sapiens (mya) | Accession number | Sequence length | Whole sequence identity | C-terminus identity |
| Homo sapiens | Human | Primates | 0 | NP_699177.2 | 799 | 1.000 | 1.000 |
| Pongo abelii | Orangutan | Primates | 15.76 | -- | 784 | 0.921 | 0.854 |
| Macaca nemestrina | Southern pig-tailed macaque | Primates | 29.44 | XP_011733709.1 | 823 | 0.694 | 0.828 |
| Vicugna pacos | Alpaca | Artiodactyla | 96 | XP_015106214.1 | 740 | 0.433 | 0.512 |
| Ceratotherium simum simum | White rhinoceros | Perissodactyla | 96 | XP_014646569.1 | 864 | 0.412 | 0.527 |
| Loxodonta africana | African bush elephant | Proboscidea | 105 | XP_010594135.1 | 829 | 0.382 | 0.489 |
| Canis lupus familiaris | Dog | Carnivora | 96 | XP_013967473.1 | 900 | 0.362 | 0.445 |
| Ailuropoda melanoleuca | Giant panada | Carnivora | 96 | XP_019665441.1 | 852 | 0.353 | 0.460 |
| Rhinolophus sinicus | Chinese horseshoe bat | Chiroptera | 96 | XP_019610944.1 | 808 | 0.345 | 0.459 |
| Dasypus novemcinctus | Nine-banded armadillo | Cingulata | 105 | XP_012377569.1 | 886 | 0.342 | 0.500 |
| Trichechus manatus latirostris | Manatee | Sirenia | 105 | XP_012412857.1 | 950 | 0.335 | 0.475 |
| Chrysochloris asiatica | Cape golden mole | Afrosoricida | 105 | XP_006835746.1 | 683 | 0.330 | 0.443 |
| Oryctolagus cuniculus | European rabbit | Lagomorpha | 90 | XP_017205124.1 | 811 | 0.305 | 0.438 |
| Monodelphis domestica | Gray short-tailed opossum | Didelphimorphia | 159 | XP_007500895.1 | 728 | 0.303 | 0.443 |
| Ornithorhynchus anatinus | Platypus | Monotremata | 177 | XP_007668655.1 | 715 | 0.302 | 0.429 |
| Gavialis gangeticus | Fish-eating crocodile | Crocodilia | 312 | XP_019380828.1 | 697 | 0.309 | 0.458 |
| Chelonia mydas | Green sea turtle | Testudines | 312 | XP_007070584.1 | 749 | 0.297 | 0.453 |
| Apteryx australis mantelli | North Island brown kiwi | Apterygiformes | 312 | XP_013807123.1 | 647 | 0.295 | 0.444 |
| Columba livia | Rock dove | Columbiformes | 312 | XP_005509980.1 | 668 | 0.287 | 0.442 |
| Pygoscelis adeliae | Adelie penguin | Sphenisciformes | 312 | XP_009323754.1 | 657 | 0.282 | 0.458 |
| Nanorana parkeri | Tibet frog | Anura | 352 | XP_018417228.1 | 586 | 0.260 | 0.376 |
Function
BEND2 is predicted to be a DNA-binding protein due to the presence of BEN domains at its C-terminus, a hypothesis supported by its localization to the nucleus, the transcription factors found in its promoter region, and the nature of the proteins it interacts with. Though the precise function of the BEND2 protein is not yet well understood by the scientific community, BEN domains have been found to be important regulators of transcription.[20]
Clinical significance
The diseases that have been linked to BEND2 are related to the central nervous system though expression of the gene is not highly observed in these tissues.
- BEND2 was identified as one of the genes that causes a central nervous system primitive neuroectodermal tumor when fused with the MN1 gene, which is located on chromosome 22.[28][29]
- A rare primary central nervous system lymphoma tumor was found to have a high mutation ratio for BEND2; however, the authors do not describe this gene as primarily responsible for the tumor.[30]
- A young girl diagnosed with severe epileptic encephalopathy was found to have a 300-kb deletion in a region that included BEND2, an extremely rare mutation not found in her parents’ genomes.[31]
- An individual with adult autism was identified to have a copy-number variant of unknown significance in a region only containing BEND2.[32]
References
- "BEND2 BEN domain containing 2 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2017-02-03.
- mieg@ncbi.nlm.nih.gov, Danielle Thierry-Mieg and Jean Thierry-Mieg, NCBI/NLM/NIH. "AceView: Gene:BEND2, a comprehensive annotation of human, mouse and worm genes with mRNAs or ESTsAceView". www.ncbi.nlm.nih.gov. Retrieved 2017-02-03.
- "Genatlas sheet". genatlas.medecine.univ-paris5.fr. Retrieved 2017-02-03.
- "BEN domain containing 2 (BEND2)". www.ncbi.nlm.nih.gov. Retrieved 2017-02-03.
- EMBL-EBI, InterPro. "BEN domain (IPR018379) < InterPro < EMBL-EBI". www.ebi.ac.uk. Retrieved 2017-02-03.
- "BEND2 Symbol Report | HUGO Gene Nomenclature Committee". www.genenames.org. Retrieved 2017-02-03.
- Database, GeneCards Human Gene. "BEND2 Gene - GeneCards | BEND2 Protein | BEND2 Antibody". www.genecards.org. Retrieved 2017-02-03.
- "BEND2 BEN domain containing 2 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2017-02-21.
- Roy A, Kucukural A, Zhang Y (April 2010). "I-TASSER: a unified platform for automated protein structure and function prediction". Nature Protocols. 5 (4): 725–38. doi:10.1038/nprot.2010.5. PMC 2849174. PMID 20360767.
- "Statistical analysis of protein sequence (SAPS)". SDSC Biology Workbench- Protein Tools. Retrieved 4 April 2017.
- "AAStats". SDSC Biology Workbench- Protein Tools. Retrieved 4 April 2017.
- "PI". SDSC Biology Workbench- Protein Tools. Retrieved 4 April 2017.
- "BEN domain-containing protein 2 isoform 1 [Homo sapiens] - Protein - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2017-02-03.
- "PSORT: Protein Subcellular Localization Prediction Too". GenScript. 24 November 1999.
- Boisvert M, Bouchard-Lévesque V, Fernandes S, Tijssen P (October 2014). "Classic nuclear localization signals and a novel nuclear localization motif are required for nuclear transport of porcine parvovirus capsid proteins". Journal of Virology. 88 (20): 11748–59. doi:10.1128/JVI.01717-14. PMC 4178750. PMID 25078698.
- "NetAcet, NetPhos, GPS, GPS-Lipids, GPS-SUMOlyation, and NetGlycate". ExPASY.
- Lim J, Hao T, Shaw C, Patel AJ, Szabó G, Rual JF, et al. (May 2006). "A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration". Cell. 125 (4): 801–14. doi:10.1016/j.cell.2006.03.032. PMID 16713569.
- "IntAct- search for BEND2". EMBL-EBI. Retrieved 19 April 2017.
- Oláh J, Vincze O, Virók D, Simon D, Bozsó Z, Tõkési N, et al. (September 2011). "Interactions of pathological hallmark proteins: tubulin polymerization promoting protein/p25, beta-amyloid, and alpha-synuclein". The Journal of Biological Chemistry. 286 (39): 34088–100. doi:10.1074/jbc.M111.243907. PMC 3190826. PMID 21832049.
- Dai Q, Ren A, Westholm JO, Serganov AA, Patel DJ, Lai EC (March 2013). "The BEN domain is a novel sequence-specific DNA-binding domain conserved in neural transcriptional repressors". Genes & Development. 27 (6): 602–14. doi:10.1101/gad.213314.113. PMC 3613608. PMID 23468431.
- "GDS3113 / 227904". www.ncbi.nlm.nih.gov. Retrieved 2017-04-25.
- National Center for Biotechnology Information. "EST Profile - Hs.403802". www.ncbi.nlm.nih.gov. Retrieved 2017-04-25.
- "ElDorado: Annotation and Analysis". Genomatix. 2017.
- "Human genome, search for BEND2 isoform 1 protein". BLAT.
- "BEN domain containing (BEND) Gene Family | HUGO Gene Nomenclature Committee". www.genenames.org. Retrieved 2017-02-21.
- "Homo sapiens BEN domain containing 2 (BEND2), transcript variant 1, mR - Nucleotide - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2017-02-21.
- "Gene: BEND2 (ENSG00000177324) - Summary - Homo sapiens - Ensembl genome browser 87". useast.ensembl.org. Retrieved 2017-02-21.
- PDQ Pediatric Treatment Editorial Board (2002-01-01). "Childhood Cancer Genomics (PDQ®): Health Professional Version". PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US). PMID 27466641.
- Sturm D, Orr BA, Toprak UH, Hovestadt V, Jones DT, Capper D, et al. (February 2016). "New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs". Cell. 164 (5): 1060–1072. doi:10.1016/j.cell.2016.01.015. PMC 5139621. PMID 26919435.
- Fukumura K, Kawazu M, Kojima S, Ueno T, Sai E, Soda M, et al. (June 2016). "Genomic characterization of primary central nervous system lymphoma". Acta Neuropathologica. 131 (6): 865–75. doi:10.1007/s00401-016-1536-2. PMID 26757737.
- Bahi-Buisson N, Girard B, Gautier A, Nectoux J, Fichou Y, Saillour Y, et al. (January 2010). "Epileptic encephalopathy in a girl with an interstitial deletion of Xp22 comprising promoter and exon 1 of the CDKL5 gene". American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 153B (1): 202–7. doi:10.1002/ajmg.b.30974. PMID 19455595.
- Stobbe G, Liu Y, Wu R, Hudgings LH, Thompson O, Hisama FM (January 2014). "Diagnostic yield of array comparative genomic hybridization in adults with autism spectrum disorders". Genetics in Medicine. 16 (1): 70–7. doi:10.1038/gim.2013.78. PMID 23765050.