Lancelet
The lancelets (/ˈlænslɪts/ or /ˈlɑːnslɪts/), also known as amphioxi (singular: amphioxus /æmfiˈɒksəs/), consist of some 30–35 species of "fish-like" benthic filter feeding chordates [2] in the order Amphioxiformes. They are the modern representatives of the subphylum Cephalochordata. Lancelets closely resemble, and are believed to be related to, 530-million-year-old Pikaia, fossils of which are known from the Burgess Shale. Zoologists are interested in them because they provide evolutionary insight into the origins of vertebrates. Lancelets contain many organs and organ systems that are closely related to those of modern fish, but in more primitive form. Therefore, they provide a number of examples of possible evolution exaptation. For example, the gill-slits of lancelets are used for feeding only, and not for respiration. The circulatory system carries food throughout their body, but does not have red blood cells or hemoglobin for transporting oxygen. Lancelet genomes hold clues about the early evolution of vertebrates, by comparing genes from lancelets with the same genes in vertebrates, the change in gene function as vertebrates evolved can be discovered.[3] The genome of a few species in the genus Branchiostoma have been sequenced: B. floridae, [4] B. belcheri,[5] B. lanceolatum. [6]
| Lancelet | |
|---|---|
 | |
| Branchiostoma lanceolatum | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Subphylum: | Cephalochordata |
| Class: | Leptocardii Müller, 1845 |
| Order: | Amphioxiformes Obscure, 1886 |
| Families | |
| Synonyms | |
|
Branchiostomiformes[1] | |
In Asia, lancelets are harvested commercially as food for humans and domesticated animals. In Japan, amphioxus (B. belcheri) has been listed in the registry of “Endangered Animals of Japanese Marine and Fresh Water Organisms.” [7]
Ecology
Habitat
Lancelets are distributed in shallow subtidal sand flats in temperate (as far north as Norway[8]), subtropical and tropical seas around the world.[9] The only exception is Asymmetron inferum, a species known from the vicinity of whale falls at a depth of about 225 m (738 ft).[10] Although they are able to swim, adult amphioxi are mostly benthic. They live in sandy bottoms whose granulometry depends on the species and the site,[11] and they are usually found half-buried in sand.[9] When disturbed, they quickly leave their burrow, swim a short distance, and then rapidly burrow again, posterior end first, into the sand. Adults (B. floridae) can tolerate salinities as low as 6‰ and temperatures from 3 to 37 °C.[12]
Feeding
Their habitat preference reflects their feeding method: they only expose the front end to the water and filter-feed on plankton by means of a branchial ciliary current that passes water through a mucous sheet. Branchiostoma floridae is capable of trapping particles from microbial to small phytoplankton size,[13] while B. lanceolatum preferentially traps bigger particles (>4 µm).[14]
Reproduction and spawning
Lancelets are gonochoric animals, i.e. having two sexes, and reproduce via external fertilization. They only reproduce during their spawning season, which varies slightly between species - usually corresponding to spring and summer months.[11] All lancelets species spawn shortly after sunset, either synchronously (e.g. Branchiostoma floridae, about once every 2 weeks during spawning season[12]) or asynchronously (Branchiostoma lanceolatum, gradual spawning through the season[15]).
Nicholas and Linda Holland were the first researchers to describe a method of obtaining amphioxus embryos by induction of spawning in captivity and in vitro fertilization.[16] Spawning can be artificially induced in the lab by electric or thermal shock.[17]
History
Taxonomy
The first representative organism of the group to be described was Branchiostoma lanceolatum. It was described by Peter Simon Pallas in 1774 as molluscan slugs in the genus Limax. It was not until 1834 that Gabriel Costa brought the phylogenetic position of the group closer to the agnathan vertebrates (hagfish and lampreys), including it in the new genus Branchiostoma (from the Greek, branchio = "gills", stoma = "mouth").[18] In 1836, Yarrel renamed the genus as Amphioxus (from the Greek: "pointed on both sides"), now considered an obsolete synonym of the genus Branchiostoma. Today, the term "amphioxus" is still used as a common name for the Amphioxiformes, along with "lancelet," especially in the English language. The order Amphioxiformes was apparently named in 1886 in the Jahresbericht und Abhandlungen des Naturwissenschaftlichen Vereins in Magdeburg.[19][20]
Anatomy
Observations of amphioxus anatomy began in the middle of the 19th century. First, the adult then the embryonic anatomy were described.[21]
Alexander Kowalevsky first described the key anatomical features of the adult amphioxus (hollow dorsal nerve tube, endostyle, segmented body, postanal tail).[21] De Quatrefages first completely described the nervous system of amphioxus.[22] Other important contributions to amphioxus adult anatomy were given by Heinrich Rathke [23] and John Goodsir.[24]
Kowalevsky also released the first complete description of amphioxus embryos,[21] while Schultze and Leuckart were the first to describe the larvae.[25] Other important contributions to amphioxus embryonic anatomy were given by Hatschek, Conklin[26] and later by Tung (experimental embryology).[27]
Anatomy
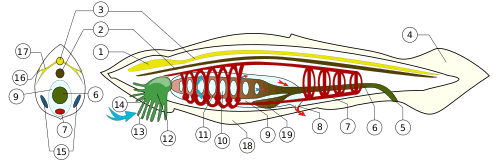
Depending on the exact species involved, the maximum length of lancelets is typically 2.5 to 8 cm (1.0–3.1 in).[10][28] Branchiostoma belcheri and B. lanceolatum are among the largest.[10] Except for the size, the species are very similar in general appearance, differing mainly in the number of myotomes and the pigmentation of their larvae.[10] They have a translucent, somewhat fish-like body, but without any paired fins or other limbs. A relatively poorly developed tail fin is present, so they are not especially good swimmers. While they do possess some cartilage-like material stiffening the gill slits, mouth, and tail, they have no true skeleton.[29]
Nervous system
In common with vertebrates, lancelets have a hollow nerve cord running along the back, pharyngeal slits and a tail that runs past the anus. Also like vertebrates, the muscles are arranged in blocks called myomeres.
Unlike vertebrates, the dorsal nerve cord is not protected by bone but by a simpler notochord made up of a cylinder of cells that are closely packed to form a toughened rod. The lancelet notochord, unlike the vertebrate spine, extends into the head. This gives the subphylum its name (κεφαλή, kephalē means 'head'). The nerve cord is only slightly larger in the head region than in the rest of the body, so that lancelets do not appear to possess a true brain. However, developmental gene expression and transmission electron microscopy indicate the presence of a diencephalic forebrain, a possible midbrain, and a hindbrain[30][31] but recent studies involving a comparison with vertebrates indicates that the vertebrate thalamus, pretectum, and midbrain domains jointly correspond to a single amphioxus region, which has been termed Di-Mesencephalic primordium (DiMes)[32]
Visual system
Lancelets have four known kinds of light-sensing structures: Joseph cells, Hesse organs, an unpaired anterior eye and lamellar body, all of which utilize opsins as light receptors. All of these organs and structures are located in the neural tube, with the frontal eye at the front, followed by the lamellar body, the Joseph cells, and the Hesse organs.[33][10][34]
Joseph cells and Hesse organs
Joseph cells are bare photoreceptors surrounded by a band of microvilli. These cells bear the opsin melanopsin. The Hesse organs (also known as dorsal ocelli) consist of a photoreceptor cell surrounded by a band of microvilli and bearing melanopsin, but half enveloped by a cup-shaped pigment cell. The peak sensitivity of both cells is ~470 nm[35] (blue).
Both the Joseph cells and Hesse organs are in the neural tube, the Joseph cells forming a dorsal column, the Hesse organs in the ventral part along the length of the tube. The Joseph cells extend from the caudal end of the anterior vesicle (or cerebral vesicle) to the boundary between myomeres 3 and 4, where the Hesse organs begin and continue nearly to the tail.[36][37]
Frontal eye
The frontal eye consists of a pigment cup, a group of putative photoreceptor cells (termed Row 1), three rows of neurons (Rows 2–4), and glial cells. The frontal eye, which expresses the PAX6 gene, has been proposed as the homolog of vertebrate paired eyes, the pigment cup as the homolog of the RPE (retinal pigment epithelium), the putative photoreceptors as homologs of vertebrate rods and cones, and Row 2 neurons as homologs of the retinal ganglion cells.[38]
The pigment cup is oriented concave dorsally. Its cells contain the pigment melanin.[38][39]
The putative photoreceptor cells, Row 1, are arranged in two diagonal rows, one on either side of the pigment cup, symmetrically positioned with respect to the ventral midline. The cells are flask-shaped, with long, slender ciliary processes (one cilium per cell). The main bodies of the cells lie outside of the pigment cup, while the cilia extend into the pigment cup before turning and exiting. The cells bear the opsin c-opsin 1, except for a few which carry c-opsin 3.[38][40]
The Row 2 cells are serotonergic neurons in direct contact with Row 1 cells. Row 3 and 4 cells are also neurons. Cells of all four rows have axons that project into the left and right ventrolateral nerves. For Row 2 neurons, axon projections have been traced to the tegmental neuropil. The tegmental neuropil has been compared with locomotor control regions of the vertebrate hypothalamus, where paracrine release modulates locomotor patterns such as feeding and swimming.[38]
Feeding and digestive system
Lancelets are inactive filter feeders,[41] spending most of the time half-buried in sand with only their frontal part protruding.[42] They eat a wide variety of small planktonic organisms, such as bacteria, fungi, diatoms, dinoflagellates and zooplankton, and they will also take detritus.[9] Little is known about the diet of the lancelet larvae in the wild, but captive larvae of several species can be maintained on a diet of phytoplankton, although this apparently is not optimal for Asymmetron lucayanum.[9]
Lancelets have oral cirri, thin tentacle-like strands that hang in front of the mouth and act as sensory devices and as a filter for the water passing into the body. Water passes from the mouth into the large pharynx, which is lined by numerous gill-slits. The ventral surface of the pharynx contains a groove called the endostyle, which, connected to a structure known as Hatschek's pit, produces a film of mucus. Ciliary action pushes the mucus in a film over the surface of the gill slits, trapping suspended food particles as it does so. The mucus is collected in a second, dorsal groove, known as the epipharyngeal groove, and passed back to the rest of the digestive tract. Having passed through the gill slits, the water enters an atrium surrounding the pharynx, then exits the body via the atriopore.[29]
Both adults and larvae exhibit a "cough" reflex to clear the mouth or throat of debris or items too large to swallow. In larvae the action is mediated by the pharyngeal muscles while in the adult animal it is accomplished by atrial contraction.[43][44]
The remainder of the digestive system consists of a simple tube running from the pharynx to the anus. The hepatic caecum, a single blind-ending caecum, branches off from the underside of the gut, with a lining able to phagocytize the food particles, a feature not found in vertebrates. Although it performs many functions of a liver, it is not considered a true liver but a homolog of the vertebrate liver.[45][46][47]
Other systems
Lancelets have no respiratory system, breathing solely through their skin, which consists of a simple epithelium. Despite the name, little if any respiration occurs in the “gill” slits, which are solely devoted to feeding. The circulatory system does resemble that of primitive fish in its general layout, but is much simpler, and does not include a heart. There are no blood cells, and no hemoglobin.[29]
The excretory system consists of segmented "kidneys" containing protonephridia instead of nephrons, and quite unlike those of vertebrates. Also unlike vertebrates, there are numerous, segmented gonads.[29]
Model organism
Lancelets became famous in the 1860s when Ernst Haeckel ("the German Darwin") began promoting them as a model for the ancestor of all vertebrates. By 1900 lancelets had become a model organism. By the mid-20th century they had fallen out of favor for a variety of reasons, including a decline of comparative anatomy and embryology, and due to the belief that lancelets were more derived than they appeared, e.g., the profound asymmetry in the larval stage.[48][49]
With the advent of molecular genetics lancelets are once again regarded as a model of vertebrate ancestors, and are used again as a model organism.[50][18]
As a result of their use in science, methods of keeping and breeding lancelets in captivity have been developed for several of the species, initially the European Branchiostoma lanceolatum, but later also the West Pacific Branchiostoma belcheri and Branchiostoma japonicum, the Gulf of Mexico and West Atlantic Branchiostoma floridae and the circumtropical (however, genetic evidence suggest the Atlantic and Indo-Pacific populations should be recognized as separate[41]) Asymmetron lucayanum.[9][51] They can reach an age of up to 7–8 years.[51]
Taxonomy
The Cephalochordata is traditionally seen as a sister subphylum to the vertebrates, with which it is grouped together into a clade (sometimes called Notochordata) which in turn is the sister group to the simpler still Urochordata. Newer research suggests this is not the case. The Cephalochordata is the most basal subphylum of the chordates,[54] while the sister group of the vertebrates are the tunicates, previously known as the urochordates.[55] However, older molecular studies[56] placed cephalochordates nearer to vertebrates, and "[m]ost authors regard amphioxus as the closest relative of the Vertebrata on the basis of 10–15 [morphological] features that are not seen in tunicates".[56]
Among the three extant (living) genera, Asymmetron is basal and it is placed in its own family. Genetic studies have come to separate conclusions on their divergence, with some suggesting that Asymmetron diverged from other lancelets more than 100 million years ago and others (with higher statistic support) that it occurred about 42 million years ago.[41] The two remaining genera, Branchiostoma and Epigonichthys diverged from each other about 36 million years ago.[41] Despite this deep separation, hybrids between Asymmetron lucayanum and Branchiostoma floridae are viable (among the deepest split species known to be able to produce such hybrids).[9]
The following are the species recognised by ITIS. Other sources recognize about thirty species.[49][41][57] It is likely that currently unrecognized cryptic species remain.[9]
- Order Branchiostomiformes
- Family Asymmetronidae
- Genus Asymmetron Andrews 1893 [Amphioxides Gill 1895]
- Asymmetron inferum Nishikawa 2004
- Asymmetron lucayanum Andrews 1893 (Sharptail lancelet)
- Genus Asymmetron Andrews 1893 [Amphioxides Gill 1895]
- Family Epigonichthyidae Peters 1876
- Genus Epigonichthys Peters 1876 [Amphipleurichthys Whitley 1932; Bathyamphioxus Whitley 1932; Heteropleuron Kirkaldy 1895; Merscalpellus Whitley 1932; Notasymmetron Whitley 1932; Paramphioxus Haekel 1893; Zeamphioxus Whitley 1932]
- Epigonichthys australis (Raff 1912)
- Epigonichthys bassanus (Günther 1884)
- Epigonichthys cingalensis (Kirkaldy 1894); nomen dubium[58]
- Epigonichthys cultellus Peters 1877
- Epigonichthys hectori (Benham 1901) (Hector's lancelet)
- Epigonichthys maldivensis (Foster Cooper 1903)
- Genus Epigonichthys Peters 1876 [Amphipleurichthys Whitley 1932; Bathyamphioxus Whitley 1932; Heteropleuron Kirkaldy 1895; Merscalpellus Whitley 1932; Notasymmetron Whitley 1932; Paramphioxus Haekel 1893; Zeamphioxus Whitley 1932]
- Family Branchiostomidae Bonaparte 1841
- Genus Branchiostoma Costa 1834 non Newport 1845 non Banks 1905 [Amphioxus Yarrell 1836; Limax Pallas 1774 non Linnaeus 1758 non Férussac 1819 non Martyn 1784; Dolichorhynchus Willey 1901 non Mulk & Jairajpuri 1974]
- Branchiostoma africae Hubbs 1927
- Branchiostoma arabiae Webb 1957
- Branchiostoma bazarutense Gilchrist 1923
- Branchiostoma belcheri Gray 1847 (Belcher's lancelet)
- Branchiostoma bennetti Boschung & Gunter 1966 (Mud lancelet)
- Branchiostoma bermudae Hubbs 1922
- Branchiostoma californiense Andrews 1893 (Californian lancelet)
- Branchiostoma capense Gilchrist 1902
- Branchiostoma caribaea Sundevall 1853 (Caribbean lancelet)
- Branchiostoma clonasea
- Branchiostoma elongata Sundevall 1852
- Branchiostoma floridae Hubbs 1922 (Florida lancelet)
- Branchiostoma gambiense Webb 1958
- Branchiostoma indica Willey 1901
- Branchiostoma japonica Willey 1896 (Pacific lancelet)
- Branchiostoma lanceolata Pallas 1774 (European lancelet)
- Branchiostoma leonense Webb 1956
- Branchiostoma longirostra Boschung 1983 (Shellhash lancelet)
- Branchiostoma malayana Webb 1956
- Branchiostoma moretonense Kelly 1966; nomen dubium[59][60]
- Branchiostoma nigeriense Webb 1955
- Branchiostoma platae Hubbs 1922
- Branchiostoma senegalense Webb 1955
- Branchiostoma tattersalli Hubbs 1922
- Branchiostoma virginiae Hubbs 1922 (Virginian lancelet)
- Genus Branchiostoma Costa 1834 non Newport 1845 non Banks 1905 [Amphioxus Yarrell 1836; Limax Pallas 1774 non Linnaeus 1758 non Férussac 1819 non Martyn 1784; Dolichorhynchus Willey 1901 non Mulk & Jairajpuri 1974]
- Family Asymmetronidae
References
- GBIF. "Species Search Results for browse/resource/344/taxon/7498708". Archived from the original on 2015-07-16. Retrieved 2009-09-25.
- Poss, Stuart G.; Boschung, Herbert T. (1996-01-01). "Lancelets (cephalochordata: Branchiostomattdae): How Many Species Are Valid?". Israel Journal of Zoology. 42 (sup1): S13–S66. doi:10.1080/00212210.1996.10688872 (inactive 2020-03-26). ISSN 0021-2210.
- Worm-like Marine Animal Providing Fresh Clues About Human Evolution Newswise, Retrieved on July 8, 2008.
- Rokhsar, Daniel S.; Satoh, Nori; Holland, Peter W. H.; Holland, Linda Z.; Fujiyama, Asao; Bronner-Fraser, Marianne; Toyoda, Atsushi; Shin-I, Tadasu; Schmutz, Jeremy (2008). "The amphioxus genome and the evolution of the chordate karyotype". Nature. 453 (7198): 1064–1071. Bibcode:2008Natur.453.1064P. doi:10.1038/nature06967. ISSN 1476-4687. PMID 18563158.
- Xu, Anlong; Chen, Shangwu; Dong, Meiling; Wu, Fenfang; Fu, Yonggui; Yuan, Shaochun; You, Leiming; Zhou, Sisi; Qiujin Zhang (2014-12-19). "Decelerated genome evolution in modern vertebrates revealed by analysis of multiple lancelet genomes". Nature Communications. 5: 5896. Bibcode:2014NatCo...5.5896H. doi:10.1038/ncomms6896. ISSN 2041-1723. PMC 4284660. PMID 25523484.
- Escriva, Hector; Petillon, Yann Le; Belgacem, Mohamed R.; Bertrand, Stephanie; Oulion, Silvan (2012-05-09). "Sequencing and Analysis of the Mediterranean Amphioxus (Branchiostoma lanceolatum) Transcriptome". PLOS One. 7 (5): e36554. Bibcode:2012PLoSO...736554O. doi:10.1371/journal.pone.0036554. ISSN 1932-6203. PMC 3348903. PMID 22590565.
- Tomiyama, Minoru; Azuma, Nobuyuki; Kubokawa, Kaoru (1998). "A New Population of the Amphioxus (Branchiostoma belcheri) in the Enshu-Nada Sea in Japan". Zoological Science. 15 (5): 799–803. doi:10.2108/zsj.15.799. ISSN 0289-0003.
- Tambs-Lyche, H. (1967). "Branchiostoma lanceolatum (Pallas) in Norway". Sarsia. 29 (1): 177–182. doi:10.1080/00364827.1967.10411078.
- Carvalho, J.E.; F. Lahaye; M. Schubert (2017). "Keeping amphioxus in the laboratory: an update on available husbandry methods". Int. J. Dev. Biol. 61 (10–11–12): 773–783. doi:10.1387/ijdb.170192ms. PMID 29319123.
- Wanninger, Andreas (11 August 2015). Evolutionary Developmental Biology of Invertebrates 6: Deuterostomia. Springer. pp. 93–94, 108–109. ISBN 978-3-7091-1856-6. Retrieved 21 November 2015.
- Escriva, Hector (2018). "My Favorite Animal, Amphioxus: Unparalleled for Studying Early Vertebrate Evolution" (PDF). BioEssays. 40 (12): 1800130. doi:10.1002/bies.201800130. ISSN 1521-1878. PMID 30328120.
- Stokes, M. Dale; Holland, Nicholas D. (1996). "Reproduction of the Florida Lancelet (Branchiostoma floridae): Spawning Patterns and Fluctuations in Gonad Indexes and Nutritional Reserves". Invertebrate Biology. 115 (4): 349. doi:10.2307/3227024. ISSN 1077-8306. JSTOR 3227024.
- Smith, Allison J.; Nash, Troy R.; Ruppert, Edward E. (2000). "The size range of suspended particles trapped and ingested by the filter-feeding lancelet Branchiostoma floridae (Cephalochordata: Acrania)". Journal of the Marine Biological Association of the United Kingdom. 80 (2): 329–332. doi:10.1017/S0025315499001903. ISSN 1469-7769.
- Riisgard, Hans Ulrik; Svane, Ib (1999). "Filter Feeding in Lancelets (Amphioxus), vertebrate Biology". 118 (4): 423. doi:10.2307/3227011. ISSN 1077-8306. JSTOR 3227011. Cite journal requires
|journal=(help) - Fuentes, Michael; Benito, Elia; Bertrand, Stephanie; Paris, Mathilde; Mignardot, Aurelie; Godoy, Laura; Jimenez‐Delgado, Senda; Oliveri, Diana; Candiani, Simona (2007). "Insights into spawning behavior and development of the european amphioxus (Branchiostoma lanceolatum)". Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 308B (4): 484–493. doi:10.1002/jez.b.21179. ISSN 1552-5015. PMID 17520703.
- NICHOLAS D. HOLLAND and LINDA Z. HOLLAND (1989). "Fine Structural Study of the Cortical Reaction and Formation of the Egg Coats in a Lancelet". The Biological Bulletin. 176 (2): 111–122. doi:10.2307/1541578. JSTOR 1541578.
- Guang Li, ZongHuang Shu, Yiquan Wang (2013). "Year-Round Reproduction and Induced Spawning of Chinese Amphioxus, Branchiostoma belcheri, in Laboratory". PLOS One. 8 (9): e75461. Bibcode:2013PLoSO...875461L. doi:10.1371/journal.pone.0075461. PMC 3784433. PMID 24086537.CS1 maint: multiple names: authors list (link)
- Garcia-Fernàndez, Jordi; Benito-Gutierrez, Èlia (June 2008). "It's a long way from amphioxus: descendants of the earliest chordate". BioEssays. 31 (6): 665–675. doi:10.1002/bies.200800110. PMID 19408244.
- Naturwissenschaftlicher Verein in Magdeburg.; Magdeburg, Naturwissenschaftlicher Verein in (1886). Jahresbericht und Abhandlungen des Naturwissenschaftlichen Vereins in Magdeburg. 1886. Magdeburg: The Verein.
- "Amphioxiformes". zoobank.org. Retrieved 2020-03-07.
- Kowalevsky AO. (1867). Entwickelungsgeschichte des Amphioxus lanceolatus. Mém Acad Sci St Petersburg.
- de Quatrefages, M. A. (1845). Annales des sciences naturelles. Libraires‐editeurs.
- H. Rathke (1841). Bemerkungen uber den Bau des Amphioxus lanceolatus eines Fisches aus der Ordnung der Cyclostomen. Gebrüder Bornträger.
- J. Goodsir (1844). Proc. R. Soc. Edinb.
- M. Schultze (1851). Z. Wiss. Zool.
- B. Hatschek (1893). "The Amphioxus and Its Development". Nature. 48 (1252): 613. Bibcode:1893Natur..48..613E. doi:10.1038/048613a0. hdl:2027/hvd.hn25lj.
- S. Yan. Dev. Growth. Differ. 1999.
- Barnes, M.K.S (7 June 2015). Tyler-Walters, H.; K. Hiscock (eds.). "Lancelet (Branchiostoma lanceolatum)". Marine Life Information Network: Biology and Sensitivity Key Information Reviews. Retrieved 7 January 2018.
- Romer, Alfred Sherwood; Parsons, Thomas S. (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 18–21. ISBN 978-0-03-910284-5.
- Candiani, Simona; Moronti, Luca; Ramoino, Paola; Schubert, Michael; Pestarino, Mario (2012). "A neurochemical map of the developing amphioxus nervous system". BMC Neuroscience. 13 (1): 59. doi:10.1186/1471-2202-13-59. ISSN 1471-2202. PMC 3484041. PMID 22676056.
- Holland, L. Z. (2015). "The origin and evolution of chordate nervous systems". Philosophical Transactions of the Royal Society B: Biological Sciences. 370 (1684): 20150048. doi:10.1098/rstb.2015.0048. ISSN 0962-8436. PMC 4650125. PMID 26554041.
- Albuixech-Crespo B, López-Blanch L, Burguera D, Maeso I, Sánchez-Arrones L, et al. (2017). "Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain". PLOS Biology. 15 (4): e2001573. doi:10.1371/journal.pbio.2001573. PMC 5396861. PMID 28422959.
- Nieuwenhuys, Rudolf; ten Donkelaar, Hans J.; Charles Nicholson (14 November 2014). The Central Nervous System of Vertebrates. Springer. p. 371. ISBN 978-3-642-18262-4. Retrieved 25 November 2015.
- Lamb, Trevor D. (2013). "Evolution of phototransduction, vertebrate photoreceptors and retina". Progress in Retinal and Eye Research. 36: 52–119. doi:10.1016/j.preteyeres.2013.06.001. ISSN 1350-9462. PMID 23792002.
- del Pilar Gomez, M.; Anyfgueyra, J. M.; Nasi, E. (2009). "Light-transduction in melanopsin-expressing photoreceptors of Amphioxus". Proceedings of the National Academy of Sciences. 106 (22): 9081–9086. Bibcode:2009PNAS..106.9081D. doi:10.1073/pnas.0900708106. ISSN 0027-8424. PMC 2690026. PMID 19451628.
- Le Douarin, Nicole Marthe; Dupin, Elisabeth (23 November 2013). Paul Trainor (ed.). Neural Crest Cells: Evolution, development and disease. Academic Press. p. 10. ISBN 978-0-12-404586-6. Retrieved 25 November 2015.
- Wicht, Helmut; Lacalli, Thurston C. (2005). "The nervous system of amphioxus: Structure, development, and evolutionary significance". Canadian Journal of Zoology. 83 (1): 122–150. doi:10.1139/z04-163. ISSN 0008-4301.
- Vopalensky, P.; Pergner, J.; Liegertova, M.; Benito-Gutierrez, E.; Arendt, D.; Kozmik, Z. (18 September 2012). "Molecular analysis of the amphioxus frontal eye unravels the evolutionary origin of the retina and pigment cells of the vertebrate eye". Proceedings of the National Academy of Sciences. 109 (38): 15383–15388. Bibcode:2012PNAS..10915383V. doi:10.1073/pnas.1207580109. ISSN 0027-8424. PMC 3458357. PMID 22949670.
- Jankowski, Roger (19 March 2013). The Evo-Devo Origin of the Nose, Anterior Skull Base and Midface. Springer Science & Business Media. p. 152. ISBN 978-2-8178-0422-4. Retrieved 7 December 2015.
- Lacalli, T. C. (29 March 1996). "Frontal Eye Circuitry, Rostral Sensory Pathways and Brain Organization in Amphioxus Larvae: Evidence from 3D Reconstructions" (PDF). Philosophical Transactions of the Royal Society B: Biological Sciences. 351 (1337): 243–263. Bibcode:1996RSPTB.351..243L. doi:10.1098/rstb.1996.0022. ISSN 0962-8436. Archived from the original (PDF) on 21 October 2018. Retrieved 14 December 2015.
- Igawa, T.; M. Nozawa; D.G. Suzuki; J.D. Reimer; A.R. Morov; Y. Wang; Y. Henmi; K. Yasui (2017). "Evolutionary history of the extant amphioxus lineage with shallow-branching diversification". Scientific Reports. 7 (1): 1157. Bibcode:2017NatSR...7.1157I. doi:10.1038/s41598-017-00786-5. PMC 5430900. PMID 28442709.
- Kotpal, R.L. (2008–2009). Modern Text Book of Zoology: Vertebrates (3 ed.). p. 76. ISBN 978-81-7133-891-7.
- Rogers, Lesley J.; Andrew, Richard (25 March 2002). Comparative Vertebrate Lateralization. Cambridge University Press. pp. 72 ff. ISBN 978-1-139-43747-9.
- Rigon, Francesca; Stach, Thomas; Caicci, Federico; Gasparini, Fabio; Burighel, Paolo; Manni, Lucia (2013). "Evolutionary diversification of secondary mechanoreceptor cells in tunicata". BMC Evolutionary Biology. 13 (1): 112. doi:10.1186/1471-2148-13-112. ISSN 1471-2148. PMC 3682859. PMID 23734698.
- Yuan, Shaochun; Ruan, Jie; Huang, Shengfeng; Chen, Shangwu; Xu, Anlong (February 2015). "Amphioxus as a model for investigating evolution of the vertebrate immune system" (PDF). Developmental & Comparative Immunology. 48 (2): 297–305. doi:10.1016/j.dci.2014.05.004. ISSN 0145-305X. PMID 24877655. Archived from the original (PDF) on 22 December 2015. Retrieved 16 December 2015.
- Yu, Jr-Kai Sky; Lecroisey, Claire; Le Pétillon, Yann; Escriva, Hector; Lammert, Eckhard; Laudet, Vincent (2015). "Identification, Evolution and Expression of an Insulin-Like Peptide in the Cephalochordate Branchiostoma lanceolatum". PLOS One. 10 (3): e0119461. Bibcode:2015PLoSO..1019461L. doi:10.1371/journal.pone.0119461. ISSN 1932-6203. PMC 4361685. PMID 25774519.
- Escriva, Hector; Chao, Yeqing; Fan, Chunxin; Liang, Yujun; Gao, Bei; Zhang, Shicui (2012). "A Novel Serpin with Antithrombin-Like Activity in Branchiostoma japonicum: Implications for the Presence of a Primitive Coagulation System". PLOS ONE. 7 (3): e32392. Bibcode:2012PLoSO...732392C. doi:10.1371/journal.pone.0032392. ISSN 1932-6203. PMC 3299649. PMID 22427833.
- Hopwood, Nick (January 2015). "The cult of amphioxus in German Darwinism; or, Our gelatinous ancestors in Naples' blue and balmy bay". History and Philosophy of the Life Sciences. 36 (3): 371–393. doi:10.1007/s40656-014-0034-x. ISSN 0391-9714. PMC 4286652. PMID 26013195.
- Tudge, Colin (2000). The Variety of Life. Oxford University Press. ISBN 0198604262.
- Holland, L.Z.; Laudet, V.; Schubert, M. (September 2004). "The chordate amphioxus: an emerging model organism for developmental biology". Cellular and Molecular Life Sciences. 61 (18): 2290–2308. doi:10.1007/s00018-004-4075-2. ISSN 1420-682X. PMID 15378201.
- "Amphioxus Branchiostoms lanceolatum". EMBRC France. Retrieved 7 January 2018.
- Gewin, V (2005). "Functional genomics thickens the biological plot". PLOS Biology. 3 (6): e219. doi:10.1371/journal.pbio.0030219. PMC 1149496. PMID 15941356.
- Lancelet (amphioxus) genome and the origin of vertebrates Ars Technica, 19 June 2008.
- Gee, Henry (19 June 2008). "Evolutionary biology: The amphioxus unleashed". Nature. 453 (7198): 999–1000. Bibcode:2008Natur.453..999G. doi:10.1038/453999a. PMID 18563145.
- Putnam, N. H.; Butts, T.; Ferrier, D. E. K.; Furlong, R. F.; Hellsten, U.; Kawashima, T.; Robinson-Rechavi, M.; Shoguchi, E.; Terry, A.; Yu, J. K.; Benito-Gutiérrez, E. L.; Dubchak, I.; Garcia-Fernàndez, J.; Gibson-Brown, J. J.; Grigoriev, I. V.; Horton, A. C.; De Jong, P. J.; Jurka, J.; Kapitonov, V. V.; Kohara, Y.; Kuroki, Y.; Lindquist, E.; Lucas, S.; Osoegawa, K.; Pennacchio, L. A.; Salamov, A. A.; Satou, Y.; Sauka-Spengler, T.; Schmutz, J.; Shin-i, T. (Jun 2008). "The amphioxus genome and the evolution of the chordate karyotype". Nature. 453 (7198): 1064–1071. Bibcode:2008Natur.453.1064P. doi:10.1038/nature06967. ISSN 0028-0836. PMID 18563158.
- Michael J. Benton (2005). Vertebrate Palaeontology, Third Edition 8. Oxford: Blackwell Publishing. ISBN 0-632-05637-1.
- WoRMS Editorial Board (2013). "World Register of Marine Species- Cephalochordates species list". Retrieved 2013-10-22. Cite journal requires
|journal=(help) - "WoRMS - World Register of Marine Species - Epigonichthys Peters, 1876".
- "UNESCO-IOC Register of Marine Organisms (URMO) - Branchiostoma mortonense Kelly, 1966".
- "WoRMS - World Register of Marine Species - Branchiostoma mortonense Kelly, 1966".
Further reading
- Stach, T. G. (2004). Cephalochordata (Lancelets). In M. Hutchins, R. W. Garrison, V. Geist, P. V. Loiselle, N. Schlager, M. C. McDade, ...W. E. Duellman (Eds.), Grzimek's Animal Life Encyclopedia (2nd ed., Vol. 1, pp. 485–493). Detroit: Gale.
- Stokes, M. D. and Holland, N. D. 1998. American Scientist 86: 552-560
External links
| Wikimedia Commons has media related to Amphioxiformes. |
| Wikispecies has information related to Cephalochordata |
- Branchiostoma japonicum and B. belcheri are Distinct Lancelets (Cephalochordata) in Xiamen Waters in China – article at Research Gate
- Error in the Genealogy of Human
- A special issue of Amphioxus Research (I)
- A special issue of Amphioxus Research (II)
- Amphioxus and the T-box gene
- A movie of the amphioxus embryonic development on YouTube
- Scripps Scientists Discover Fluorescence in Key Marine Creature
- Amphioxus at GeoChemBio: taxonomy, brief facts, life cycle and embryology
- View the braFlo1 genome assembly in the UCSC Genome Browser.
