Alizarin Red S
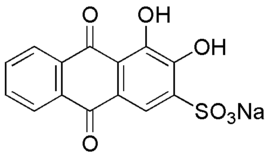
Alizarin Red S (also known as C.I. Mordant Red 3, Alizarin Carmine, and C.I 58005[1]. It is a water-soluble sodium salt of Alizarin sulfonic acid with a chemical formula of C
14H
7NaO
7S.[2][1] Alizarin Red S was discovered by Graebe and Libermann in 1871.[2] In the field of histology alizarin Red S is been used to stain calcium deposits in tissues,[3][4] and in geology to stain and differentiate carbonate minerals.[3]
 | |
| Names | |
|---|---|
| IUPAC name
3,4-Dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.530 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C14H7NaO7S | |
| Molar mass | 342.253 g/mol |
| Appearance | yellow-orange powder |
| Soluble in water and ethanol | |
| Hazards | |
| Safety data sheet | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses

Alizarin Red S is used in histology and histopathology to stain, or locate calcium deposits in tissues.[1][3][4] In the presence of calcium, Alizarin Red S, binds to the calcium to form a Lake pigment that is orange to red in color.[4] Whole specimens can be stained with Alizarin Red S to show the distribution of bone, especially in developing embryos.[4] In living corals alizarin Red S has been used to mark daily growth layers.[5]
In geology, Alizarin Red S is used on Thin sections, and polished surfaces to help identify carbonate minerals which stain at different rates.[6]
See also
- Aniline
- 1,2,4-Trihydroxyanthraquinone or purpurin, another red dye that occurs in madder root
- Hydroxyanthraquinone
- Dihydroxyanthraquinone
- List of dyes
- List of colors (compact)
References
- Lillie, Ralph Dougall (1977). H. J. Conn's Biological stains (9th ed.). Baltimore: Williams & Wilkins. pp. 692p.
- Legan, Lea; Retko, Klara; Ropret, Polonca (2016). "Vibrational spectroscopic study on degradation of alizarin carmine". Microchemical Journal. 127: 36–45. doi:10.1016/j.microc.2016.02.002. ISSN 0026-265X.
- Puchtler, Holde; Meloan, Susan N.; Terry, Mary S. (1969). "On the history and mechanism of alizarin and alizarin red S stains for calcium". Journal of Histochemistry & Cytochemistry. 17 (2): 110–124. doi:10.1177/17.2.110. ISSN 0022-1554. PMID 4179464.
- Bancroft, John; Stevens, Alan, eds. (1982). The Theory and Practice of Histological Techniques (2nd ed.). Longman Group Limited.
- Holcomb, Michael; Cohen, Anne L.; McCorkle, Daniel C. (2013). "An evaluation of staining techniques for marking daily growth in scleractinian corals" (PDF). Journal of Experimental Marine Biology and Ecology. 440: 126–131. doi:10.1016/j.jembe.2012.12.003. hdl:1912/5857. ISSN 0022-0981.
- Dickson, J. A. D. (1966). "Carbonate identification and genesis as revealed by staining". Journal of Sedimentary Research. 36 (4): 491–505. doi:10.1306/74D714F6-2B21-11D7-8648000102C1865D.
