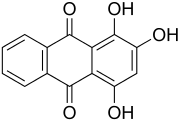
1,2,4-Trihydroxyanthraquinone
1,2,4-Trihydroxyanthraquinone, commonly called purpurin, is an anthraquinone. It is a naturally occurring red/yellow dye. It is formally derived from 9,10-anthraquinone by replacement of three hydrogen atoms by hydroxyl (OH) groups.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,2,4-trihydroxyanthracene-9,10-dione | |
| Other names
Purpurin(e), Hydroxylizaric acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.237 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H8O5 | |
| Molar mass | 256.21 g/mol |
| Melting point | 259 °C (498 °F; 532 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Purpurin is also called verantin, smoke Brown G, hydroxylizaric acid, and C.I. 58205. It is a minor component of the classical lake pigment "madder lake" or Rose Madder.
History
Madder root has been used for dying cloth at least since 1500 BC.[2] Purpurin and alizarin were isolated from the root by Pierre Robiquet and Colin, two French chemists, in 1826. They were identified as anthracene derivatives by Gräbe and Liebermann in 1868. They also synthesized alizarin from bromoanthraquinone, which, together with the conversion of alizarin into purpurin published previously by M. F. De Lalande, provided the first synthetic route to purpurin.[3] The positions of the OH groups were determined by Bayer and Caro in 1874–1875.[4]
Natural occurrence
Purpurin occurs in the roots of the madder plant (Rubia tinctorum), together with alizarin (1,2-dihydroxyanthraquinone). The root actually contains colorless glycosides of the dyes.
Properties
Purpurin is a crystalline solid, that forms orange needles melting at 259 °C (498 °F),[5] but becomes red when dissolved in ethanol, and yellow when dissolved with alkalis in boiling water. It is insoluble in hexane but soluble in chloroform, and can be obtained from chloroform as reddish needles.[6] Unlike alizarin, purpurin is dissolved by boiling in a solution of aluminum sulfate, from which it can be precipitated by acid. This procedure can be used to separate the two dyes.[7]
Like many dihydroxy- and trihydroxyanthraquinones, pupurin has a purgative action, although only 1/20 as effective as 1,2,7-trihydroxyanthraquinone (anthrapurpurin).[8]
Uses
Purpurin is a fast dye for cotton printing and forms complexes with various metal ions. However it fades faster than alizarin on exposure to sunlight.[2]
A study published in Nature journal Scientific Reports suggests that the purpurin could replace cobalt in lithium-ion batteries.[9] Eliminating cobalt would mean eliminating a hazardous material, allow batteries to be produced at room temperature, and lower the cost of recycling batteries. Extracting purpurin from farmed madder is a simple task; alternately, the chemical could be synthesized in a lab.[10]
See also
- Trihydroxyanthraquinone
- Pierre Robiquet, discoverer of purpurin in 1826
References
- CRC Handbook of Chemistry & Physics, 90th Ed.
- Madder Root Archived 2011-07-14 at the Wayback Machine catalog entry at Natural Pigments website. Accessed on 2010-01-22.
- Chemical news and journal of industrial science, Volume 30, Page 207
- Wahl, Andre; Atack, F. W (1919) The Manufacture Of Organic Dyestuffs. G. Bell And Sons, Limited. Online version accessed on 2010-01-22.
- CRC Handbook of Chemistry & Physics, 90th Ed.
- Vankar, Padma S.; Shanker, Rakhi; Mahanta, Debajit; Tiwari, S.C. (2008). "Ecofriendly sonicator dyeing of cotton with Rubia cordifolia Linn. using biomordant". Dyes and Pigments. 76 (1): 207–212. doi:10.1016/j.dyepig.2006.08.023.
- Irving Wetherbee Fay (1919) The chemistry of the coal-tar dyes. Van Nostrand Online version accessed on 2010-01-22.
- Hugh Alister McGuigan (1921), An introduction to chemical pharmacology; pharmacodynamics in relation to chemistry. P. Blakiston's son, Philadelphia. Online version at archive.org, accessed on 2010-01-30.
- Reddy, Arava Leela Mohana; Nagarajan, Subbiah; Chumyim, Porramate; Gowda, Sanketh R; Pradhan, Padmanava; Jadhav, Swapnil R; Dubey, Madan; John, George; Ajayan, Pulickel M; Ajayan, Pulickel M (2012). "Lithium storage mechanisms in purpurin based organic lithium ion battery electrodes". Scientific Reports. 2: 960. doi:10.1038/srep00960. PMC 3518813. PMID 23233879.
- Richard Chirgwin (12 December 2012). "Dying to make greener batteries". The Register. Retrieved 12 December 2012.