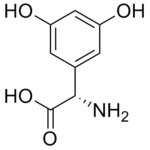
Dihydroxyphenylglycine
(S)-3,5-Dihydroxyphenylglycine or DHPG is a potent agonist of group I metabotropic glutamate receptors (mGluRs) mGluR1 and mGluR5.
 | |
| Names | |
|---|---|
| IUPAC name
(S)-2-amino-2-(3,5-dihydroxyphenyl)acetic acid | |
| Other names
3,5-dihydroxyphenylglycine, DHPG, S-DHPG | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| MeSH | 3,5-dihydroxyphenylglycine |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H9N1O4 | |
| Molar mass | 183.05 g mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
DHPG was the first agonist shown to be selective for group I mGluRs.[1] Agonist activity is found in only the (S)-isomer, and (S)-DHPG may be a partial agonist of group I mGluRs.[1]
(S)-DHPG has been investigated for therapeutic effects in the treatment of neuronal injury (such as those associated with ischemia or hypoxia), cognitive enhancement, and Alzheimer's disease.[1]
3,5-Dihydroxyphenylglycine can be isolated from the latex of Euphorbia helioscopia.[2]
DHGP is also found in vancomycin and related glycopeptides. Although the (S) stereoisomer is synthesized by the DpgA-D enzymes,[3] it is the (R) stereoisomer that is used in vancomycin and other related compounds. DHPG is enzymatically derived from the polyketide synthase pathway.
Biosynthesis
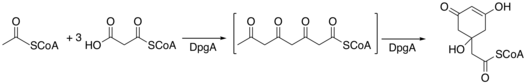
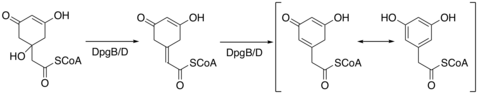
When synthesized in bacteria, DHPG requires 5 enzymes, DpgA-D and 4-hydroxyphenylglycine transferase (Pgat), in order to be synthesized.[4] DpgA is a type III polyketide synthase and initiates the synthesis by condensing acetyl-CoA with three molecules of malonyl-CoA. The tetra-carbonyl compound then cyclizes to form a C8 intermediate. DpgB/D then dehydrates the intermediate using enolate chemistry to promote the loss of water. DpgB/D isomerizes the product to aromatize the ring.
DpgC oxidizes the aromatic intermediate at the benzylic carbon using oxygen to an alpha-keto compound. DpgC performs this oxidation in absence of any iron, heme, flavin, or pterin cofactors. Chen et al suggest the following reaction mechanism to explain the reactivity of DpgC.[5] This mechanism is supported by findings reported in Widboom et al in 2007.[6] Finally, the molecule is transaminated by 4-hydroxyphenylglycine transferase using tyrosine to become DHPG.
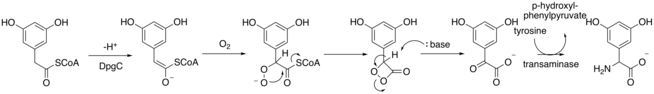
4-Hydroxyphenylglycine transferase synthesizes the (S) stereoisomer of DHPG, however, an epimerase switches the stereocenter to the (R) configuration after DHPG is incorporated into the vancomycin non-ribosomal polypeptide.
References
- Wiśniewski K.; Car, H. (2002). "(S)-3,5-DHPG: a review". CNS Drug Rev. 8 (1): 101–116. PMID 12070529.
- Müller, P.; Schütte, H. R. (May 1968). "m-Hydroxyphenylglycine and 3,5-dihydroxyphenylglycine, 2 new amino acids from the latex of Euphorbia helioscopia". Z. Naturforsch. B (in German). 23 (5): 659–663. PMID 4385921.
- Yim, G., Thaker, M. N., Koteva, K., Wright, G. "Glycopeptide antibiotic biosynthesis." The Journal of Antibiotics, 2017, 67, 31-41.
- Pfeifer, V., Nicholson, G. J., Ries, J., Recktenwalk, J., Schefer, A. B., Shawky, R. M., Schröder, J., Wohlleben, W., Pelzer, S. "A Polyketide Synthase in glycopeptide Biosynthesis: the Biosynthesis of the Non-Proteogenic Amino Acid (S)-3,5-Dihydroxyphenylglycine." The Journal of Biological Chemistry, 2001, 276 (42/19), 38370-38377.
- Chen, H., Tseng, C. C., Hubbard, B. K., Walsh, C. T. "Glycopeptide antibiotic biosyntehsis: Enzymatic assembly of the dedicated amino acid monomy (S)-3,5-dihydroxyphenylglycine." PNAS, 2001, 98 (26), 14901-14906.
- Widboom, P. F., Fielding, E. N., Liu, Y., Bruner, S. D. "Structural basis for cofactor-independent dioxygenation in vancomycin biosynthesis." Nature, 2007, 447, 342-345.