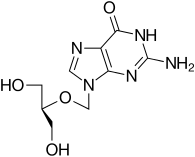
Ganciclovir
Ganciclovir is an antiviral medication used to treat cytomegalovirus (CMV) infections. A prodrug form with improved oral bioavailability (valganciclovir) has also been developed.
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɡænˈsaɪkləvɪər/ |
| Trade names | Cytovene; Cymevene; Vitrasert |
| Other names | ganciclovir (INN, USAN, BAN); gancyclovir; DHPG; 9-(1,3-dihydroxy-2-propoxymethyl)guanine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605011 |
| Pregnancy category | |
| Routes of administration | IV, oral, intravitreal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 5% (oral) |
| Metabolism | guanylate kinase (CMV UL97 gene product) |
| Elimination half-life | 2.5–5 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.155.403 |
| Chemical and physical data | |
| Formula | C9H13N5O4 |
| Molar mass | 255.234 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 250 °C (482 °F) (dec.) |
| |
| |
| (verify) | |
Ganciclovir was patented in 1980 and approved for medical use in 1988.[1]
Medical use
Ganciclovir is indicated for:[2]
- Sight-threatening CMV retinitis in severely immunocompromised people
- CMV pneumonitis in bone marrow transplant recipients
- Prevention of CMV disease in bone marrow and solid organ transplant recipients
- Confirmed CMV retinitis in people with AIDS (intravitreal implant)
It is also used for acute CMV colitis in HIV/AIDS and CMV pneumonitis in immunosuppressed patients.
Ganciclovir has also been used with some success in treating Human herpesvirus 6 infections.[3]
Ganciclovir has also been found to be an effective treatment for herpes simplex virus epithelial keratitis.[4]
Adverse effects
Ganciclovir is commonly associated with a range of serious haematological adverse effects. Common adverse drug reactions (≥1% of patients) include: granulocytopenia, neutropenia, anaemia, thrombocytopenia, fever, nausea, vomiting, dyspepsia, diarrhea, abdominal pain, flatulence, anorexia, raised liver enzymes, headache, confusion, hallucination, seizures, pain and phlebitis at injection site (due to high pH), sweating, rash, itch, increased serum creatinine and blood urea concentrations.[2]
Toxicity
Ganciclovir is considered a potential human carcinogen, teratogen, and mutagen. It is also considered likely to cause inhibition of spermatogenesis. Thus, it is used judiciously and handled as a cytotoxic drug in the clinical setting.[2][5]
Mechanism of action
Ganciclovir is a synthetic analogue of 2′-deoxy-guanosine. It is first phosphorylated to ganciclovir monophosphate by a viral kinase encoded by the cytomegalovirus (CMV) gene UL97 during infection. Subsequently, cellular kinases catalyze the formation of ganciclovir diphosphate and ganciclovir triphosphate, which is present in 10-fold greater concentrations in CMV or herpes simplex virus (HSV)-infected cells than uninfected cells.
Ganciclovir triphosphate is a competitive inhibitor of deoxyguanosine triphosphate (dGTP) incorporation into DNA and preferentially inhibits viral DNA polymerases more than cellular DNA polymerases. In addition, ganciclovir triphosphate serves as a poor substrate for chain elongation, thereby disrupting viral DNA synthesis by a second route.
Pharmacokinetics
Absorption of the oral form is very limited—about 5% fasting, about 8% with food. It achieves a concentration in the central nervous system of about 50% of the plasma level. About 90% of plasma ganciclovir is eliminated unchanged in the urine, with a half-life of 2–6 hours, depending on renal function (elimination takes over 24 hours in end-stage renal disease).
Administration
Acute infections are treated in two phases:
- induction phase, 5 mg per kilogram intravenously every 12 hours for 14–21 days, the intravenous dose given as a 1-hour infusion
- maintenance phase, 5 mg per kg intravenously every day
Stable disease is treated with 1000 mg orally three times daily. Similar dosing is used to prevent disease in high-risk patients, such as those infected with human immunodeficiency virus (HIV) or those with organ transplants.
Ganciclovir is also available in slow-release formulations for insertion into the vitreous humour of the eye, as treatment for CMV retinitis (associated with HIV infection).
A topical ophthalmic gel preparation of ganciclovir was recently approved for the treatment of acute herpes simplex keratitis.
References
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 504. ISBN 9783527607495.
- Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- Nakano (2009). "Detection and identification of U69 gene mutations encoded by ganciclovir-resistant human herpesvirus 6 using denaturing high-performance liquid chromatography". Cite journal requires
|journal=(help) - Wilhelmus KR (January 2015). "Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis". The Cochrane Database of Systematic Reviews. 1: CD002898. doi:10.1002/14651858.CD002898.pub5. PMC 4443501. PMID 25879115.
- Roche Products Pty Ltd. Cymevene (Australian Approved Product Information). Dee Why (NSW): Roche; 2005.
Further reading
- Noble S, Faulds D (July 1998). "Ganciclovir. An update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients". Drugs. 56 (1): 115–46. doi:10.2165/00003495-199856010-00012. PMID 9664203.
- Spector SA (1999). "Oral ganciclovir". Advances in Experimental Medicine and Biology. Advances in Experimental Medicine and Biology. 458: 121–7. doi:10.1007/978-1-4615-4743-3_11. ISBN 978-1-4613-7150-2. PMID 10549384.
- Couchoud-Heyer C (July 2007). "WITHDRAWN: Cytomegalovirus prophylaxis with antiviral agents for solid organ transplantation". The Cochrane Database of Systematic Reviews (4): CD001320. doi:10.1002/14651858.cd001320.pub2. PMID 17636667.