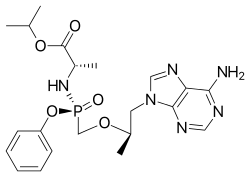
Tenofovir alafenamide
Tenofovir alafenamide, sold under the brand name Vemlidy, is a hepatitis B virus (HBV) nucleotide reverse transcriptase inhibitor for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease.[2] It is taken by mouth.[2]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌtəˈnoʊfəvɪər ˌæləˈfɛnəmaɪd/ |
| Trade names | Vemlidy Genvoya (with elvitegravir, cobicistat and emtricitabine) Odefsey (with emtricitabine and rilpivirine) Descovy (with emtricitabine) Symtuza (with darunavir, cobicistat, and emtricitabine) |
| Other names | GS-7340 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category | |
| Routes of administration | Oral (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | ~80%[2] |
| Elimination half-life | 0.51 hour |
| Excretion | Feces (31.7%), urine (<1%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H29N6O5P |
| Molar mass | 476.474 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
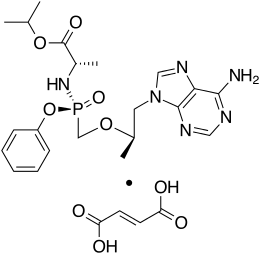
Tenofovir alafenamide is a prodrug of tenofovir. It was developed by Gilead Sciences based on the protide technology of Chris McGuigan for use in the treatment of HIV/AIDS and chronic hepatitis B, and is applied in the form of tenofovir alafenamide fumarate (TAF). Closely related to the commonly used reverse-transcriptase inhibitor tenofovir disoproxil fumarate (TDF), TAF has greater antiviral activity and better distribution into lymphoid tissues than that agent.[3][4] Vemlidy was approved by the U.S. Food and Drug Administration (FDA) in November 2016.[5]
Gilead announced a Phase III clinical trial evaluating a single-tablet regimen combining tenofovir alafenamide with cobicistat, emtricitabine and elvitegravir[6] and developed a coformulation of the drug with cobicistat, emtricitabine and the protease inhibitor darunavir.[7][8][9] In a 48-week study comparing elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil (trade name Stribild) to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (trade name Genvoya), the results showed the newer drug to be noninferior to the established agent, but at much lower dosages and with lower incidence of adverse side effects such as impaired kidney function.[10][11][12] The FDA approved the TAF-based treatment regimen for treatment of HIV-1 in November 2015.[13] Genvoya is the first TAF-based regimen to receive approval.[13]
Fixed-dose combinations containing tenofovir alafenamide
- Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (brand name Genvoya)[14] — approved both in the United States and in the European Union in November 2015[15][16] (compare Elvitegravir/cobicistat/emtricitabine/tenofovir; brand name Stribild[17][18][19])
- Emtricitabine/rilpivirine/tenofovir alafenamide (brand name Odefsey)[20] — approved in the United States in March 2016, and in the European Union in June 2016[21][22] (compare Emtricitabine/rilpivirine/tenofovir; brand name Complera[23][24])
- Emtricitabine/tenofovir alafenamide (brand name Descovy)[25] — approved in the United States in April 2016 (compare Emtricitabine/tenofovir; brand name Truvada). In October 2019, Descovy was approved in the United States for HIV-1 pre-exposure prophylaxis (PrEP).[26][27]
- Bictegravir/emtricitabine/tenofovir alafenamide (brand name Biktarvy)[28] — approved in the United States in February 2018.
- Darunavir/cobicistat/emtricitabine/tenofovir alafenamide (brand name Symtuza)[29] — approved in the United States in July 2018
See also
- Sofosbuvir—an HCV RNA polymerase (NS5B) inhibitor bearing structural similarity (phosphoramidate side chain metabolized by cathepsin A, carboxylesterase 1 and histidine triad nucleotide-binding protein 1)[30][31]
References
- "Tenofovir alafenamide (Vemlidy) Use During Pregnancy". Drugs.com. 26 December 2018. Retrieved 18 April 2020.
- "Vemlidy- tenofovir alafenamide tablet". DailyMed. 11 February 2020. Retrieved 18 April 2020.
- Eisenberg EJ, He GX, Lee WA (2001). "Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood". Nucleosides Nucleotides Nucleic Acids. 20 (4–7): 1091–8. doi:10.1081/NCN-100002496. PMID 11562963.
- M Markowitz, A Zolopa, et al. GS-7340 Demonstrates Greater Declines in HIV-1 RNA than Tenofovir Disoproxil Fumarate During 14 Days of Monotherapy in HIV-1 Infected Subjects. 18th Conference on Retroviruses and Opportunistic Infections 2 Mar 2011. Paper # 152LB
- "FDA Approves Vemlidy (tenofovir alafenamide) for Chronic Hepatitis B in Adults". United States Department of Health and Human Services. 21 November 2016. Archived from the original on 11 October 2019. Retrieved 11 October 2019.
- "Gilead Initiates Phase 3 Clinical Program for Tenofovir Alafenamide, a Novel Low-Dose Prodrug for the Treatment of HIV" (Press release). Gilead. 24 January 2013. Archived from the original on 11 October 2019.
- "Gilead Sciences Finalizes Agreement with Tibotec Pharmaceuticals to Develop and Commercialize a Single-Tablet Regimen of Prezista with Emtriva, GS 7340 and Cobicistat". Gilead Sciences (Press release). 15 November 2011. Archived from the original on 11 October 2019. Retrieved 10 October 2019.
- GS-7340 Packs Greater HIV Punch, Potentially Better Safety, Versus Viread Horn, Tim. 15 Mar 2012. AIDSmeds.com
- Pharmacokinetics of a Novel EVG/COBI/FTC/GS-7340 Single Tablet Regimen. 13th International Workshop on Clinical Pharmacology of HIV Therapy. Barcelona, Spain. April 16–18, 2012.
- Once-Daily Tenofovir Prodrug Combo Pill as Effective as Stribild. AIDSmeds.com 1 Nov 2012.
- CROI 2013: New Pro-drug Tenofovir Alafenamide Appears Equally Effective but Better Tolerated. Highleyman, Liz. HIVandHepatitis.com. 6 March 2013.
- Horn, T. et al. Tenefovir Alafenamide Fumarate (TAF) Sign-On Letter to Gilead. 13 June 2013. Treatment Action Group.
- "U.S. Food and Drug Administration Approves Gilead's Single Tablet Regimen Genvoya (Elvitegravir, Cobicistat, Emtricitabine and Tenofovir Alafenamide) for Treatment of HIV-1 Infection" (Press release). Gilead. 5 November 2015. Archived from the original on 8 November 2015.
- "Genvoya- elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide tablet". DailyMed. 11 February 2019. Retrieved 18 April 2020.
- "Genvoya (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) fixed-dose combination tablet". U.S. Food and Drug Administration (FDA). 8 December 2015. Retrieved 28 July 2020. Lay summary (PDF).
- "Genvoya EPAR". European Medicines Agency (EMA). Retrieved 28 July 2020.
- "Drug Approval Package: Stribild (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate) Fixed Dose". U.S. Food and Drug Administration (FDA). 10 October 2012. Retrieved 28 July 2020. Lay summary (PDF).
- "Stribild- elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate tablet, film coated". DailyMed. 28 January 2019. Retrieved 28 July 2020.
- "Stribild EPAR". European Medicines Agency (EMA). Retrieved 28 July 2020.
- "Odefsey- emtricitabine, rilpivirine hydrochloride, and tenofovir alafenamide tablet". DailyMed. 6 December 2019. Retrieved 18 April 2020.
- "Odefsey (emtricitabine, rilpivirine, and tenofovir alafenamide) Tablets". U.S. Food and Drug Administration (FDA). 29 November 2016. Retrieved 28 July 2020.
- "Odefsey EPAR". European Medicines Agency (EMA). Retrieved 28 July 2020.
- "Drug Approval Package: (emtricitabine/rilpivirine/tenofovir disoproxil fumarate) NDA #202123". U.S. Food and Drug Administration (FDA). 6 September 2012. Retrieved 28 July 2020. Lay summary (PDF).
- "Complera- emtricitabine, rilpivirine hydrochloride, and tenofovir disoproxil fumarate tablet, film coated". DailyMed. 9 December 2019. Retrieved 28 July 2020.
- "Descovy- emtricitabine and tenofovir alafenamide tablet". DailyMed. 13 January 2020. Retrieved 18 April 2020.
- "FDA approves second drug to prevent HIV infection as part of ongoing efforts to end the HIV epidemic". U.S. Food and Drug Administration (FDA). 3 October 2019. Archived from the original on 11 October 2019. Retrieved 11 October 2019.
- Mandavilli, Apoorva (4 October 2019). "F.D.A. Approves New H.I.V.-Prevention Drug, but Not for Everyone". The New York Times. Retrieved 11 October 2019.
- "Biktarvy- bictegravir sodium, emtricitabine, and tenofovir alafenamide fumarate tablet". DailyMed. 8 August 2019. Retrieved 18 April 2020.
- "Symtuza- darunavir, cobicistat, emtricitabine, and tenofovir alafenamide tablet, film coated". DailyMed. 6 March 2020. Retrieved 18 April 2020.
- "Comparison of tenofovir prodrugs: TAF vs TDF". DRUG R&D INSIGHT. Retrieved 24 November 2015.
- Murakami E, Tolstykh T, Bao H, et al. (November 2010). "Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977". J. Biol. Chem. 285 (45): 34337–47. doi:10.1074/jbc.M110.161802. PMC 2966047. PMID 20801890.
External links
- "Tenofovir alafenamide". Drug Information Portal. U.S. National Library of Medicine.
- "Tenofovir alafenamide fumarate". Drug Information Portal. U.S. National Library of Medicine.