BI 224436
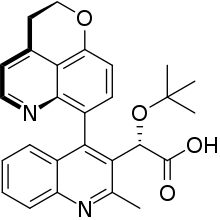
BI 224436 was an investigational new drug under development for the treatment of HIV infection. BI 224436 is the first non-catalytic site integrase inhibitor (NCINI). It inhibits HIV replication via binding to a conserved allosteric pocket of the HIV integrase enzyme. This makes the drug distinct in its mechanism of action compared to raltegravir and elvitegravir, which bind at the catalytic site.[2] In October 2011, Gilead Sciences purchased exclusive rights to develop BI 224436 and several related compounds under investigation in Boehringer Ingelheim’s noncatalytic site integrase inhibitor program.[3][4]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 7 hrs (simulated)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C27H26N2O4 |
| Molar mass | 442.515 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Clinical trials were abandoned in advance of Phase 1.[5]
References
- Pharmacodynamics of BI 224436 for HIV-1 in an in vitro hollow fiber infection model system
- Levin, Jules. BI 224436, a Non-Catalytic Site Integrase Inhibitor, is a potent inhibitor of the replication of treatment-naïve and raltegravir-resistant clinical isolates of HIV-1. Conference Reports for NATAP. ICAAC Chicago Sept 17-20 2011.
- Gilead Negotiates Worldwide License to BI’s Early Clinical Stage HIV Program. Genetic Engineering and Biotechnology News. 6 Oct 2011.
- Highleyman, Liz. ICAAC: New Integrase Inhibitor BI 224436 Active against Raltegravir-Resistant HIV. HIVandHepatitis.com. 7 Oct 2011.
- Safety and Pharmacokinetics of Multiple Rising Oral Doses of BI 224436 in Healthy Male Volunteers
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.