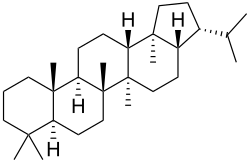
Triterpene
Triterpenes are a class of chemical compounds composed of three terpene units with the molecular formula C30H48; they may also be thought of as consisting of six isoprene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids.[1][2]
Structures
Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified.[3] These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate.
| Number of rings | Examples |
|---|---|
| 0 | Squalene |
| 1 | |
| 2 | Polypodatetraene |
| 3 | Malabaricane |
| 4 | Lanostane, Cucurbitacin |
| 5 | Hopane, Oleanane, Ursolic acid |
Triterpenoids
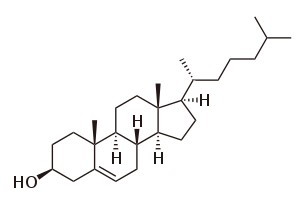
By definition triterpenes are hydrocarbons and possess no heteroatoms; functionalized triterpenes should instead be called triterpenoids. However this distinction is not always adhered to in scientific literature, with the two terms triterpene and triterpenoid often being used interchangeably.
Triterpenoids possess a rich chemistry and pharmacology (e.g. cholesterol) with several pentacyclic motifs. Lupane, oleanane and ursane show particular promise as anti-cancer agents.[4][5]
Steroids
Steroids feature a cucurbitane core, although in practice they are biosynthesised from either lanosterol (animals and fungi) or cycloartenol (plants) via the cyclization of squalene. Steroids have two principal biological functions, being either key components of cell membranes or signaling molecules that activate steroid hormone receptors. Important sub-classes include sterols and cucurbitacins.
Triterpenoid saponins
Triterpenoid saponins are triterpenes which belong to the saponin group of compounds, making them triterpenoid glycosides. They are produced by plants as part of their self-defense mechanism[6] with important sub-classes including ginsenosides[7] and eleutherosides.
Triterpenes are biosynthesized through the head-to-head condensation of two FPP units to form squalene. In turn, squalene serves as precursor for the formation of triterpenoids, including bacterial hopanoids and eukaryotic sterols. Squalene is itself a valuable compound since it is used as an antioxidant, as well as in cosmetics, nutrition, and vaccines. Squalene is extracted from shark liver oil or olive or other plant oils.[8][9]
References
- Eberhard Breitmaier (2006). "Triterpenes". Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. doi:10.1002/9783527609949.ch6. ISBN 9783527609949.
- Davis, Edward M.; Croteau, Rodney (2000). "Cyclization Enzymes in the Biosynthesis of Monoterpenes, Sesquiterpenes, and Diterpenes". Topics in Current Chemistry. 209: 53–95. doi:10.1007/3-540-48146-X_2.CS1 maint: uses authors parameter (link)
- Xu, Ran; Fazio, Gia C.; Matsuda, Seiichi P.T. (February 2004). "On the origins of triterpenoid skeletal diversity". Phytochemistry. 65 (3): 261–291. doi:10.1016/j.phytochem.2003.11.014.
- Laszczyk, Melanie (2009). "Pentacyclic Triterpenes of the Lupane, Oleanane and Ursane Group as Tools in Cancer Therapy". Planta Medica. 75 (15): 1549–60. doi:10.1055/s-0029-1186102. PMID 19742422.
- Liu, Jie (December 1995). "Pharmacology of oleanolic acid and ursolic acid". Journal of Ethnopharmacology. 49 (2): 57–68. doi:10.1016/0378-8741(95)90032-2. PMID 8847885.
- Augustin, Jörg M.; Kuzina, Vera; Andersen, Sven B.; Bak, Søren (April 2011). "Molecular activities, biosynthesis and evolution of triterpenoid saponins". Phytochemistry. 72 (6): 435–457. doi:10.1016/j.phytochem.2011.01.015. PMID 21333312.
- Attele, Anoja S; Wu, Ji An; Yuan, Chun-Su (December 1999). "Ginseng pharmacology". Biochemical Pharmacology. 58 (11): 1685–1693. doi:10.1016/S0006-2952(99)00212-9. PMID 10571242.
- Huang ZR, Lin YK, Fang JY (January 2009). "Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology". Molecules. 14 (1): 540–54. doi:10.3390/molecules14010540. PMC 6253993. PMID 19169201.
- Fox CB (September 2009). "Squalene emulsions for parenteral vaccine and drug delivery". Molecules. 14 (9): 3286–312. doi:10.3390/molecules14093286. PMC 6254918. PMID 19783926.