Trifluoroacetyl chloride
Trifluoroacetyl chloride (also known as TFAC[1]) is a gaseous chemical compound with the chemical formula C2ClF3O.[2][3] It is usually shipped as a liquid under high pressure, however.[3] The compound is a toxic gas.
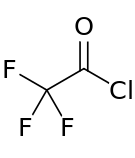 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trifluoroacetyl chloride | |||
| Other names
2,2,2-Trifluoroacetyl chloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.005.961 | ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2ClF3O | |||
| Molar mass | 132.469 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Properties
Trifluoroacetyl chloride has a vapor density that is 4.6 times that of air, or about 1.384 grams per milliliter at 20 °C (68 °F) as a liquid under pressure.[1][2] The compound has a melting point of −146 °C (−231 °F) and a boiling point of −27 °C (−17 °F).[2] The compound easily reacts with water and moist air to produce the toxic gas hydrogen chloride and trifluoroacetic acid.[3][4]
Trifluoroacetyl chloride is incompatible with a number of other varieties of chemicals, such as amines, alcohols, alkalis, and strong oxidizers. It reacts strongly with amines and alkalis. It also reacts violently with diisopropyl ether, or any ether if metal salts are present, sometimes causing an explosion.[3]
Trifluoroacetyl chloride's heat of vaporization is 20 kilojoules per mole at 65 btus per pound.[1]
Numerous atoms and compounds can replace the chlorine atom in trifluoroacetyl chloride. These include iodine, fluorine, cyanide, thiocyanate, and isocyanate. The compound also reacts easily with metal alkyls. This reaction has the form of CF3COCl + MR → CF3COR + MCl, where M can be lithium, copper, magnesium, mercury, silver, or cadmium. When trifluoroacetyl chloride also reacts with ketene and esterification yields occur, the resulting reaction forms trifluoroacetoacetate esters.[1]
Trifluoroacetyl chloride also reacts with soil, cellulose-based absorbents, and clay-based absorbents.[5] When the compound reacts with water in contact with metal, hydrogen gas, which is explosive, is produced.[6] The compound forms a clustering reaction with a methyl group (CH3).[7]
Production
Trifluoroacetyl chloride can be produced by catalytic chlorination of chlorine and trifluoroacetaldehyde.[8] The compound can also be produced if halothane is oxidized using CYP2E1.[9] This is also done with CYP2A6 instead of CPY2E1, but less readily.[10]
Applications and storage
Trifluoroacetyl chloride's applications include uses in medicine, pesticides, the fine chemical industry, and the organic intermediate industry.[3] However, the compound itself is not sold to consumers or as a commodity.[4] Some acetoacetic esters produced by trifluoroacetyl chloride are in turn used to perform chemical reactions that result in the formation of compounds with agricultural and pharmaceutical applications.[1]
One of trifluoroacetyl chloride's uses is in adding trifluoromethyl to complex molecules during chemical reactions.[1]
In the late 1970s, trifluoroacetyl chloride was explored for use as a reagent for nuclear magnetic resonance. It was intended to be used on amines, alcohols, thiols, and phenols.[11]
Trifluoroacetyl chloride is typically stored as a liquid under high pressure.[4]
Biological role, precautions, and toxicity
Liquid trifluoroacetyl chloride can cause frostbite if it comes in contact with unprotected skin. If inhaled, the compound in its gaseous state will irritate the eyes, skin, and mucous membranes. Absorbing trifluoroacetyl chloride through skin, inhaling it, or ingesting it can cause death. When the compound burns, it produces toxic gases.[3] It also corrodes the respiratory tract.[4] The compound is also a lacrimator. It can cause dyspnea if inhaled by mice, rats, or guinea pigs. A concentration of 35.3 parts per million of trifluoroacetyl chloride is enough to usually kill a rat in six hours.[6]
Trifluoroacetyl chloride does not bioaccumulate significantly. However, it is harmful to aquatic organisms.[12]
Trifluoroacetyl chloride is metabolized by Cytochrome P450 enzymes. The immune systems of organisms typically react to this.[13]
See also
References
- Trifluoroacetyl Chloride (PDF), retrieved October 14, 2013
- Trifluoroacetyl chloride, 2013, retrieved October 10, 2013
- TRIFLUOROACETYL CHLORIDE, 2010, retrieved October 10, 2013
- December 2011 Product Safety Summary of Trifluoroacetyl chloride (TFAC) (PDF), December 2011, retrieved October 14, 2013
- TRIFLUOROACETYL CHLORIDE, retrieved October 16, 2013
- Trifluoroacetyl chloride, National Institutes of Health, August 2013, retrieved October 18, 2013
- Trifluoroacetyl chloride, NIST, 2011, retrieved October 21, 2013
- Bernard Cheminal; Henri Mathais; Marc Thomarat (February 17, 1987), Process for preparing trifluoroacetyl chloride, retrieved October 18, 2013
- Arthur J. Atkinson, Jr.; Shiew-Mei Huang; Juan J.L. Lertora; Sanford P. Markey, eds. (September 18, 2012), Principles of Clinical Pharmacology, ISBN 9780123854728, retrieved October 22, 2013
- Current Concepts in Drug Metabolism and Toxicology, Academic Press, November 27, 2012, ISBN 9780123983596, retrieved October 22, 2013
- P. Sleevi; T.E. Glass; H.C. Dorn (October 1979), "Trifluoroacetyl chloride for characterization of organic functional groups by fluorine-19 nuclear magnetic resonance spectrometry", Analytical Chemistry, 51 (12): 1931–1934, doi:10.1021/ac50048a009
- Solvay Company (December 2011), Product Safety Summary of Trifluoroacetyl chloride (TFAC) (PDF), retrieved October 16, 2013
- Kevin James Coe (2008), Metabolism and Cytotoxicity of the Nitroaromatic Drug Flutamide and Its Cyano Analog in Hepatocyte Cell Lines, ProQuest, ISBN 9781109000955, retrieved October 22, 2013