Triethylammonium acetate
Triethylammonium acetate is a volatile buffering agent, which, when diluted in water, maintains pH at about 7.[1][2][3][4]
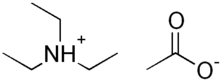 | |
| Names | |
|---|---|
| IUPAC name
Triethylammonium acetate | |
| Other names
teaa, triethylamine/acetate buffer | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.023.632 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| (CH3CH2)3NHOCOCH3 | |
| Molar mass | 161.24 g/mol |
| Boiling point | 164.5 °C (328.1 °F; 437.6 K) |
| Soluble | |
| Hazards | |
| Main hazards | Corrosive, harmful |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- "Triethylammonium Acetate, 1 M Solution - CAS 5204-74-0 - Calbiochem 625718". Sigma-Aldrich. Retrieved 2020-07-25.
- PubChem. "Triethylammonium acetate". pubchem.ncbi.nlm.nih.gov. Retrieved 2020-07-25.
- Berton, Paula; Kelley, Steven P.; Wang, Hui; Rogers, Robin D. (2018-11-01). "Elucidating the triethylammonium acetate system: Is it molecular or is it ionic?". Journal of Molecular Liquids. 269: 126–131. doi:10.1016/j.molliq.2018.08.006. ISSN 0167-7322.
- "Solution Preparation". www.chem.uci.edu. Retrieved 2020-07-25.
Acetyl halides and salts of the acetate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.