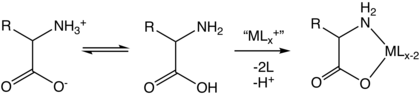
Transition metal amino acid complexes
Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the transition metals.[2] Not included in this article are complexes of the amides (including peptide) and ester derivatives of amino acids. Also excluded are the polyamino acids including the chelating agents EDTA and NTA.
Binding modes
.png)
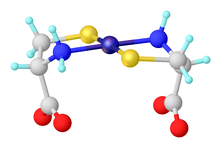
Most commonly, amino acids coordinate to metal ions as N,O bidentate ligands, utilizing the amino group and the carboxylate. They are "L-X" ligands. A five-membered chelate ring is formed. The chelate ring is only slightly ruffled at the sp3-hybridized carbon and nitrogen centers.
For those amino acids containing coordinating substituents, the resulting complexes are more structurally diverse since these substituents can coordinate. Histidine, aspartic acid, methionine, and cysteine sometimes form tridentate N,N,O, N,O,O, S,N,O, and S,N,O complexes, respectively.
Using kinetically inert metal ions, complexes containing monodentate amino acids have been characterized. These complexes exist in either the N or the O linkage isomers. It can be assumed that such monodentate complexes exist transiently for many kinetically labile metal ions (e.g. Zn2+).
Stoichiometry and structure
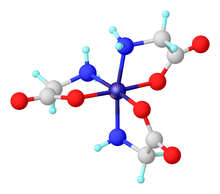
Homoleptic complexes (only amino acid ligands)
Mixing simple metal salts with solutions of amino acids near neutral or elevated pH often affords bis- or tris complexes. For metal ions that prefer octahedral coordination, these complexes often adopt the stoichiometry M(aa)3 (aa = amino carboxylate, such as glycinate, H2NCH2CO2−).
Complexes of the 3:1 stoichiometry have the formula is [M(O2CC(R)HNH2)3]z. Such complexes adopt octahedral coordination geometry. These complexes can exist in facial and meridional isomers, both of which are chiral. The stereochemical possibilities increase when the amino acid ligands are not homochiral.
Complexes with the 2:1 stoichiometry are illustrated by copper(II) glycinate [Cu(O2CC(R)HNH2)2], which exists both in anhydrous and pentacoordinate geometries. When the metal is square planar, these complexes can exist as cis and trans isomers. The stereochemical possibilities increase when the amino acid ligands are not homochiral.
Homoleptic complexes are also known where the amino carboxylate is tridentate amino acids. One such complex is Ni(κ3-histidinate)2.

Heteroleptic complexes (amino acids plus other ligands)
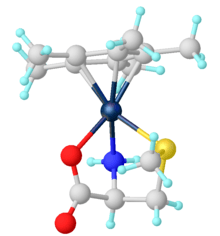
Mixed ligand complexes are common for amino acids. Well known examples include [Co(en)2(glycinate)]2+, where en (ethylenediamine) is a spectator ligand. In the area of organometallic complexes, one example of Cp*Ir(κ3-methionine).
References
- Baidya, N.; Ndreu, D.; Olmstead, M. M.; Mascharak, P. K. (1991). "Synthesis, Structure, and Properties of Potassium bis(L-cysteinato-N,S)nickelate(II) sesquihydrate". Inorganic Chemistry. 30: 2448–2451. doi:10.1021/ic00010a043.CS1 maint: multiple names: authors list (link)
- Severin, K.; Bergs, R.; Beck, W. (1998). "Bioorganometallic Chemistry-Transition Metal Complexes with α-Amino Acids and Peptides". Angew. Chem. Int. Ed. 37: 1635–1654. doi:10.1002/(SICI)1521-3773(19980703)37:12<1634::AID-ANIE1634>3.0.CO;2-C.CS1 maint: uses authors parameter (link)
- K.-Q. Gu, Y.-X. Sun, R. Zhang, N.-W. Zhang, H.-W. Che (2007). "Tris(glycinato-κ2N,O)cobalt(III)". Acta Crystallogr. E63: m740–m742. doi:10.1107/S1600536807005636.CS1 maint: uses authors parameter (link)
- A. Abbasi, B. Safarkoopayeh, N. Khosravi, A. Shayesteh (217). "Structural Studies of Bis(histidinato)nickel(II): Combined Experimental and Computational Studies". Comptes Rendus Chimie. 20: 467. doi:10.1016/j.crci.2016.12.006.CS1 maint: multiple names: authors list (link)
- M. Scharwitz, T. van Almsick, W. S. Sheldrick (2007). "(S-Methylcysteinato)(η5-pentamethylcyclopentadienyl)iridium(III) Trifluoromethanesulfonate hemihydrate". Acta Crystallogr. E63: m230-m232. doi:10.1107/S1600536806053360.CS1 maint: multiple names: authors list (link)