Sulfite ester
A sulfite ester is a functional group with the structure (RO)(R'O)SO. They adopt a trigonal pyramidal molecular geometry due to the presence of lone pairs on the sulphur atom.
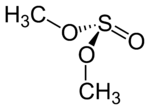
When substituents R and R' differ, the compound is chiral owing to the stereogenic sulphur centre; when the R groups are the same the compound will have idealised Cs molecular symmetry.
They are commonly prepared by the reaction of thionyl chloride with alcohols.[1] The reaction is typically performed at room temperature to prevent the alcohol being converted into a chloroalkane. Bases such as pyridine can also be used to promote the reaction:
- 2 ROH + SOCl2 → (RO)2SO + 2 HCl
The pesticide endosulfan is a sulfite ester. Some simple members include ethylene sulfite, dimethyl sulfite, and diphenylsulfite. Many examples have been prepared from diols, such as sugars. Sulfite esters can be powerful alkylation and hydroxyalkylation reagents.[2]
References
- McCormack, W. B.; Lawes, B. C. "Sulfuric and sulfurous esters" Kirk-Othmer Encycl. Chem. Technol., 3rd Ed. (1983), 22, 233-54.doi:10.1002/0471238961.1921120613030315.a01
- van Woerden, H. F. "Organic Sulfites". Chemical Reviews. 63 (6): 557–571. doi:10.1021/cr60226a001.