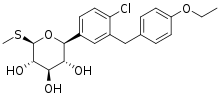
Sotagliflozin
Sotagliflozin (INN; trade name Zynquista) is a drug approved in EU for certain patients with type I diabetes.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Zynquista |
| AHFS/Drugs.com | zynquista |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.231.837 |
| Chemical and physical data | |
| Formula | C21H25ClO5S |
| Molar mass | 424.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The Food and Drug Administration refused its approval for use in combination with insulin for the treatment of type 1 diabetes. It is developed by Sanofi.[2][3]
Sotagliflozin is a sodium/glucose cotransporter 2 (SGLT2) inhibitor and is in the class of drugs known as gliflozins.
References
- Zynquista approved in EU for certain patients with type I diabetes
- "Sotagliflozin as an Adjunct to Insulin for Type 1 Diabetes - FDA" (PDF).
- Sanofi (17 January 2019). "Sanofi: FDA advisory committee votes on Zynquista(TM) (sotagliflozin) as treatment for adults with type 1 diabetes". GlobeNewswire News Room.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.