Soil pH
Soil pH is a measure of the acidity or basicity (alkalinity) of a soil. pH is defined as the negative logarithm (base 10) of the activity of hydronium ions (H+
or, more precisely, H
3O+
aq) in a solution. In soils, it is measured in a slurry of soil mixed with water (or a salt solution, such as 0.01 M CaCl
2), and normally falls between 3 and 10, with 7 being neutral. Acid soils have a pH below 7 and alkaline soils have a pH above 7. Ultra-acidic soils (pH < 3.5) and very strongly alkaline soils (pH > 9) are rare.[1][2]
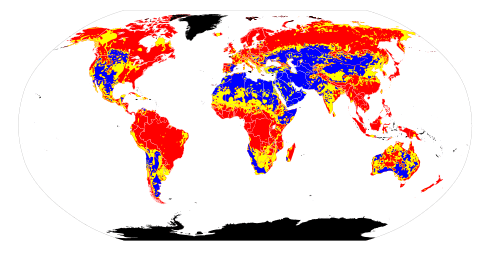
Soil pH is considered a master variable in soils as it affects many chemical processes. It specifically affects plant nutrient availability by controlling the chemical forms of the different nutrients and influencing the chemical reactions they undergo. The optimum pH range for most plants is between 5.5 and 7.5;[2] however, many plants have adapted to thrive at pH values outside this range.
Classification of soil pH ranges
The United States Department of Agriculture Natural Resources Conservation Service classifies soil pH ranges as follows: [3]
| Denomination | pH range |
|---|---|
| Ultra acidic | < 3.5 |
| Extremely acidic | 3.5–4.4 |
| Very strongly acidic | 4.5–5.0 |
| Strongly acidic | 5.1–5.5 |
| Moderately acidic | 5.6–6.0 |
| Slightly acidic | 6.1–6.5 |
| Neutral | 6.6–7.3 |
| Slightly alkaline | 7.4–7.8 |
| Moderately alkaline | 7.9–8.4 |
| Strongly alkaline | 8.5–9.0 |
| Very strongly alkaline | > 9.0 |
Determining pH
Methods of determining pH include:
- Observation of soil profile: Certain profile characteristics can be indicators of either acid, saline, or sodic conditions. Examples are:[4]
- Poor incorporation of the organic surface layer with the underlying mineral layer – this can indicate strongly acidic soils;
- The classic podzol horizon sequence, since podzols are strongly acidic: in these soils, a pale eluvial (E) horizon lies under the organic surface layer and overlies a dark B horizon;
- Presence of a caliche layer indicates the presence of calcium carbonates, which are present in alkaline conditions;
- Columnar structure can be an indicator of sodic condition.
- Observation of predominant flora. Calcifuge plants (those that prefer an acidic soil) include Erica, Rhododendron and nearly all other Ericaceae species, many birch (Betula), foxglove (Digitalis), gorse (Ulex spp.), and Scots Pine (Pinus sylvestris). Calcicole (lime loving) plants include ash trees (Fraxinus spp.), honeysuckle (Lonicera), Buddleja, dogwoods (Cornus spp.), lilac (Syringa) and Clematis species.
- Use of an inexpensive pH testing kit, where in a small sample of soil is mixed with indicator solution which changes colour according to the acidity.
- Use of litmus paper. A small sample of soil is mixed with distilled water, into which a strip of litmus paper is inserted. If the soil is acidic the paper turns red, if basic, blue.
- Use of a commercially available electronic pH meter, in which a glass or solid-state electrode is inserted into moistened soil or a mixture (suspension) of soil and water; the pH is usually read on a digital display screen.
- Recently, spectrophotometric methods have been developed to measure soil pH involving addition of an indicator dye to the soil extract.[5] These compared well to glass electrode measurements but offer substantial advantages such as lack of drift, liquid junction and suspension effects
Precise, repeatable measures of soil pH are required for scientific research and monitoring. This generally entails laboratory analysis using a standard protocol; an example of such a protocol is that in the USDA Soil Survey Field and Laboratory Methods Manual.[6] In this document the three-page protocol for soil pH measurement includes the following sections: Application; Summary of Method; Interferences; Safety; Equipment; Reagents; and Procedure.
Summary of Method— Summary of the USDA NRCS method for soil pH determination[6]The pH is measured in soil-water (1:1) and soil-salt (1:2 ) solutions. For convenience, the pH is initially measured in water and then measured in . With the addition of an equal volume of 0.02 M CaCl2 to the soil suspension that was prepared for the water pH, the final soil-solution ratio is 1:2 0.01 M .
A 20-g soil sample is mixed with 20 mL of reverse osmosis (RO) water (1:1 w:v) with occasional stirring. The sample is allowed to stand 1 h with occasional stirring. The sample is stirred for 30 s, and the 1:1 water pH is measured. The 0.02 M (20 mL) is added to soil suspension, the sample is stirred, and the 1:2 0.01 M CaCl2 pH is measured (4C1a2a2).
Factors affecting soil pH
The pH of a natural soil depends on the mineral composition of the parent material of the soil, and the weathering reactions undergone by that parent material. In warm, humid environments, soil acidification occurs over time as the products of weathering are leached by water moving laterally or downwards through the soil. In dry climates, however, soil weathering and leaching are less intense and soil pH is often neutral or alkaline.[7][8]
Sources of acidity
Many processes contribute to soil acidification. These include:[9][10]
- Rainfall: Acid soils are most often found in areas of high rainfall. Rainwater has a slightly acidic pH (usually about 5.7) due to a reaction with CO
2 in the atmosphere that forms carbonic acid. When this water flows through soil it results in the leaching of basic cations from the soil as bicarbonates; this increases the percentage of Al3+
and H+
relative to other cations. - Root respiration and decomposition of organic matter by microorganisms releases CO
2 which increases the carbonic acid (H
2CO
3) concentration and subsequent leaching. - Plant growth: Plants take up nutrients in the form of ions (e.g. NO−
3, NH+
4, Ca2+
, H
2PO−
4), and they often take up more cations than anions. However plants must maintain a neutral charge in their roots. In order to compensate for the extra positive charge, they will release H+
ions from the root. Some plants also exude organic acids into the soil to acidify the zone around their roots to help solubilize metal nutrients that are insoluble at neutral pH, such as iron (Fe). - Fertilizer use: Ammonium (NH+
4) fertilizers react in the soil by the process of nitrification to form nitrate (NO−
3), and in the process release H+
ions. - Acid rain: The burning of fossil fuels releases oxides of sulfur and nitrogen into the atmosphere. These react with water in the atmosphere to form sulfuric and nitric acid in rain.
- Oxidative weathering: Oxidation of some primary minerals, especially sulfides and those containing Fe2+
, generate acidity. This process is often accelerated by human activity:- Mine spoil: Severely acidic conditions can form in soils near some mine spoils due to the oxidation of pyrite.
- Acid sulfate soils formed naturally in waterlogged coastal and estuarine environments can become highly acidic when drained or excavated.
Sources of alkalinity
Total soil alkalinity increases with:[11][12]
- Weathering of silicate, aluminosilicate and carbonate minerals containing Na+
, Ca2+
, Mg2+
and K+
; - Addition of silicate, aluminosilicate and carbonate minerals to soils; this may happen by deposition of material eroded elsewhere by wind or water, or by mixing of the soil with less weathered material (such as the addition of limestone to acid soils);
- Addition of water containing dissolved bicarbonates (as occurs when irrigating with high-bicarbonate waters).
The accumulation of alkalinity in a soil (as carbonates and bicarbonates of Na, K, Ca and Mg) occurs when there is insufficient water flowing through the soils to leach soluble salts. This may be due to arid conditions, or poor internal soil drainage; in these situations most of the water that enters the soil is transpired (taken up by plants) or evaporates, rather than flowing through the soil.[11]
The soil pH usually increases when the total alkalinity increases, but the balance of the added cations also has a marked effect on the soil pH. For example, increasing the amount of sodium in an alkaline soil tends to induce dissolution of calcium carbonate, which increases the pH. Calcareous soils may vary in pH from 7.0 to 9.5, depending on the degree to which Ca2+
or Na+
dominate the soluble cations.[11]
Effect of soil pH on plant growth
Acid soils
Plants grown in acid soils can experience a variety of stresses including aluminium (Al), hydrogen (H), and/or manganese (Mn) toxicity, as well as nutrient deficiencies of calcium (Ca) and magnesium (Mg).[13]
Aluminium toxicity is the most widespread problem in acid soils. Aluminium is present in all soils, but dissolved Al3+ is toxic to plants; Al3+ is most soluble at low pH; above pH 5.0, there is little Al in soluble form in most soils.[14][15] Aluminium is not a plant nutrient, and as such, is not actively taken up by the plants, but enters plant roots passively through osmosis. Aluminium inhibits root growth; lateral roots and root tips become thickened and roots lack fine branching; root tips may turn brown. In the root, the initial effect of Al3+ is the inhibition of the expansion of the cells of the rhizodermis, leading to their rupture; thereafter it is known to interfere with many physiological processes including the uptake and transport of calcium and other essential nutrients, cell division, cell wall formation, and enzyme activity.[14][16]
Proton (H+ ion) stress can also limit plant growth. The proton pump, H+-ATPase, of the plasmalemma of root cells works to maintain the near-neutral pH of their cytoplasm. A high proton activity (pH within the range 3.0–4.0 for most plant species) in the external growth medium overcomes the capacity of the cell to maintain the cytoplasmic pH and growth shuts down.[17]
In soils with a high content of manganese-containing minerals, Mn toxicity can become a problem at pH 5.6 and lower. Manganese, like aluminium, becomes increasingly soluble as pH drops, and Mn toxicity symptoms can be seen at pH levels below 5.6. Manganese is an essential plant nutrient, so plants transport Mn into leaves. Classic symptoms of Mn toxicity are crinkling or cupping of leaves.
Nutrient availability in relation to soil pH
Soil pH affects the availability of some plant nutrients:
As discussed above, aluminium toxicity has direct effects on plant growth; however, by limiting root growth, it also reduces the availability of plant nutrients. Because roots are damaged, nutrient uptake is reduced, and deficiencies of the macronutrients (nitrogen, phosphorus, potassium, calcium and magnesium) are frequently encountered in very strongly acidic to ultra-acidic soils (pH<5.0).[19]
Molybdenum availability is increased at higher pH; this is because the molybdate ion is more strongly sorbed by clay particles at lower pH.[20]
Zinc, iron, copper and manganese show decreased availability at higher pH (increased sorption at higher pH).[20]
The effect of pH on phosphorus availability varies considerably, depending on soil conditions and the crop in question. The prevailing view in the 1940s and 1950s was that P availability was maximized near neutrality (soil pH 6.5–7.5), and decreased at higher and lower pH.[21][22] Interactions of phosphorus with pH in the moderately to slightly acidic range (pH 5.5–6.5) are, however, far more complex than is suggested by this view. Laboratory tests, glasshouse trials and field trials have indicated that increases in pH within this range may increase, decrease, or have no effect on P availability to plants.[22][23]
Water availability in relation to soil pH
Strongly alkaline soils are sodic and dispersive, with slow infiltration, low hydraulic conductivity and poor available water capacity.[24] Plant growth is severely restricted because aeration is poor when the soil is wet; in dry conditions, plant-available water is rapidly depleted and the soils become hard and cloddy (high soil strength).[25]
Many strongly acidic soils, on the other hand, have strong aggregation, good internal drainage, and good water-holding characteristics. However, for many plant species, aluminium toxicity severely limits root growth, and moisture stress can occur even when the soil is relatively moist.[14]
Plant pH preferences
In general terms, different plant species are adapted to soils of different pH ranges. For many species, the suitable soil pH range is fairly well known. Online databases of plant characteristics, such USDA PLANTS[26] and Plants for a Future[27] can be used to look up the suitable soil pH range of a wide range of plants. Documents like Ellenberg's indicator values for British plants[28] can also be consulted.
However, a plant may be intolerant of a particular pH in some soils as a result of a particular mechanism, and that mechanism may not apply in other soils. For example, a soil low in molybdenum may not be suitable for soybean plants at pH 5.5, but soils with sufficient molybdenum allow optimal growth at that pH.[19] Similarly, some calcifuges (plants intolerant of high-pH soils) can tolerate calcareous soils if sufficient phosphorus is supplied.[29] Another confounding factor is that different varieties of the same species often have different suitable soil pH ranges. Plant breeders can use this to breed varieties that can tolerate conditions that are otherwise considered unsuitable for that species – examples are projects to breed aluminium-tolerant and manganese-tolerant varieties of cereal crops for food production in strongly acidic soils.[30]
The table below gives suitable soil pH ranges for some widely cultivated plants as found in the USDA PLANTS Database.[26] Some species (like Pinus radiata and Opuntia ficus-indica) tolerate only a narrow range in soil pH, whereas others (such as Vetiveria zizanioides) tolerate a very wide pH range.
| Scientific name | Common name | pH (minimum) | pH (maximum) |
|---|---|---|---|
| Vetiveria zizanioides | vetivergrass | 3.0 | 8.0 |
| Pinus rigida | pitch pine | 3.5 | 5.1 |
| Rubus chamaemorus | cloudberry | 4.0 | 5.2 |
| Ananas comosus | pineapple | 4.0 | 6.0 |
| Coffea arabica | Arabian coffee | 4.0 | 7.5 |
| Rhododendron arborescens | smooth azalea | 4.2 | 5.7 |
| Pinus radiata | Monterey pine | 4.5 | 5.2 |
| Carya illinoinensis | pecan | 4.5 | 7.5 |
| Tamarindus indica | tamarind | 4.5 | 8.0 |
| Vaccinium corymbosum | highbush blueberry | 4.7 | 7.5 |
| Manihot esculenta | cassava | 5.0 | 5.5 |
| Morus alba | white mulberry | 5.0 | 7.0 |
| Malus | apple | 5.0 | 7.5 |
| Pinus sylvestris | Scots pine | 5.0 | 7.5 |
| Carica papaya | papaya | 5.0 | 8.0 |
| Cajanus cajan | pigeonpea | 5.0 | 8.3 |
| Pyrus communis | common pear | 5.2 | 6.7 |
| Solanum lycopersicum | garden tomato | 5.5 | 7.0 |
| Psidium guajava | guava | 5.5 | 7.0 |
| Nerium oleander | oleander | 5.5 | 7.8 |
| Punica granatum | pomegranate | 6.0 | 6.9 |
| Viola sororia | common blue violet | 6.0 | 7.8 |
| Caragana arborescens | Siberian peashrub | 6.0 | 9.0 |
| Cotoneaster integerrimus | cotoneaster | 6.8 | 8.7 |
| Opuntia ficus-indica | Barbary fig (pricklypear) | 7.0 | 8.5 |
Changing soil pH
Increasing pH of acidic soil
Finely ground agricultural lime is often applied to acid soils to increase soil pH (liming). The amount of limestone or chalk needed to change pH is determined by the mesh size of the lime (how finely it is ground) and the buffering capacity of the soil. A high mesh size (60 mesh = 0.25 mm; 100 mesh = 0.149 mm) indicates a finely ground lime that will react quickly with soil acidity. The buffering capacity of a soil depends on the clay content of the soil, the type of clay, and the amount of organic matter present, and may be related to the soil cation exchange capacity. Soils with high clay content will have a higher buffering capacity than soils with little clay, and soils with high organic matter will have a higher buffering capacity than those with low organic matter. Soils with higher buffering capacity require a greater amount of lime to achieve an equivalent change in pH.[31]
Amendments other than agricultural lime that can be used to increase the pH of soil include wood ash, industrial calcium oxide (burnt lime), magnesium oxide, basic slag (calcium silicate), and oyster shells. These products increase the pH of soils through various acid-base reactions. Calcium silicate neutralizes active acidity in the soil by reacting with H+ ions to form monosilicic acid (H4SiO4), a neutral solute.[32]
Decreasing the pH of alkaline soil
The pH of an alkaline soil can be reduced by adding acidifying agents or acidic organic materials. Elemental sulfur (90–99% S) has been used at application rates of 300–500 kg/ha – it slowly oxidizes in soil to form sulfuric acid. Acidifying fertilizers, such as ammonium sulfate, ammonium nitrate and urea, can help to reduce the pH of a soil because ammonium oxidises to form nitric acid. Acidifying organic materials include peat or sphagnum peat moss.[33]
However, in high-pH soils with a high calcium carbonate content (more than 2%), it can be very costly and/or ineffective to attempt to reduce the pH with acids. In such cases, it is often more efficient to add phosphorus, iron, manganese, copper and/or zinc instead, because deficiencies of these nutrients are the most common reasons for poor plant growth in calcareous soils.[34][35]
See also
References
- Slessarev, E. W.; Lin, Y.; Bingham, N. L.; Johnson, J. E.; Dai, Y.; Schimel, J. P.; Chadwick, O. A. (21 November 2016). "Water balance creates a threshold in soil pH at the global scale" (PDF). Nature. 540 (7634): 567–569. doi:10.1038/nature20139. PMID 27871089.
- Queensland Department of Environment and Heritage Protection. "Soil pH". www.qld.gov.au. Retrieved 15 May 2017.
- Soil Survey Division Staff. "Soil survey manual. 1993. Chapter 3". Soil Conservation Service. U.S. Department of Agriculture Handbook 18. Retrieved 2017-05-15.
- Buol, S. W., R. J. Southard, R.C. Graham and P.A. McDaniel. Soil Genesis and Classification. (5th) Edition, Ia. State Press p. 494. 2002
- Bargrizan S, Smernik R, Mosley LM (2017). Development of a spectrophotometric method for determining pH of soil extracts and comparison with glass electrode measurements. Soil Science Society of America Journal 81, 1350-1358. doi:10.2136/sssaj2017.04.0119
- Soil Survey Staff (2014). R. Burt and Soil Survey Staff (ed.). Kellogg Soil Survey Laboratory Methods Manual. Soil Survey Investigations Report No. 42, Version 5.0 (PDF). U.S. Department of Agriculture, Natural Resources Conservation Service. pp. 276–279. Retrieved 26 June 2017.
- USDA-NRCS. "Soil pH" (PDF). Guides for Educators: Soil Quality Kit. www.nrcs.usda.gov. Retrieved 15 May 2017.
- van Breemen, N.; Mulder, J.; Driscoll, C. T. (October 1983). "Acidification and alkalinization of soils". Plant and Soil. 75 (3): 283–308. doi:10.1007/BF02369968.
- Van Breemen, N.; Driscoll, C. T.; Mulder, J. (16 February 1984). "Acidic deposition and internal proton sources in acidification of soils and waters". Nature. 307 (5952): 599–604. doi:10.1038/307599a0.
- Sparks, Donald; Environmental Soil Chemistry. 2003, Academic Press, London, UK
- Bloom, Paul R.; Skyllberg, Ulf (2012). "Soil pH and pH buffering". In Huang, Pan Ming; Li, Yuncong; Sumner, Malcolm E. (eds.). Handbook of soil sciences : properties and processes (2nd ed.). Boca Raton, FL: CRC Press. pp. 19–1 to 19–14. ISBN 9781439803059.
- Oosterbaan, R.J. "Soil Alkalinity (Alkaline-sodic soils)" (PDF). www.waterlog.info. Retrieved 16 May 2017.
- Brady, N. and Weil, R. The Nature and Properties of Soils. 13th ed. 2002
- Kopittke, Peter M.; Menzies, Neal W.; Wang, Peng; Blamey, F. Pax C. (August 2016). "Kinetics and nature of aluminium rhizotoxic effects: a review". Journal of Experimental Botany. 67 (15): 4451–4467. doi:10.1093/jxb/erw233. PMID 27302129.
- Hansson et al (2011) Differences in soil properties in adjacent stands of Scots pine, Norway spruce and silver birch in SW Sweden. Forest Ecology and Management 262 522–530
- Rout, GR; Samantaray, S; Das, P (2001). "Aluminium toxicity in plants: a review" (PDF). Agronomie. 21 (1): 4–5. doi:10.1051/agro:2001105. Retrieved 11 June 2014.
- Shavrukov, Yuri; Hirai, Yoshihiko (January 2016). "Good and bad protons: genetic aspects of acidity stress responses in plants". Journal of Experimental Botany. 67 (1): 15–30. doi:10.1093/jxb/erv437. PMID 26417020.
- Finck, Arnold (1976). Pflanzenernährung in Stichworten. Kiel: Hirt. p. 80. ISBN 978-3-554-80197-2.
- Sumner, Malcolm E.; Yamada, Tsuioshi (November 2002). "Farming with acidity". Communications in Soil Science and Plant Analysis. 33 (15–18): 2467–2496. doi:10.1081/CSS-120014461.
- Bolan, N; Brennan, R. (2011). "Bioavailability of N, P, K, Ca, Mg, S, Si, and Micronutrients". In Huang, Pan Ming; Li, Yuncong; Sumner, Malcolm E. (eds.). Handbook of soil sciences: resource management and environmental impacts (2nd ed.). Boca Raton, FL: CRC Press. pp. 11–1 to 11–80. ISBN 9781439803073.
- Truog, Emil (1946). "The Liming of Soils". Science in farming, USDA Yearbook, 1941–1947. pp. 566–576.
- Sumner, M.E.; Farina, M.P.W. (1986). "Phosphorus interactions with other nutrients and lime in field cropping systems". In Stewart, B.A. (ed.). Advances in Soil Science. New York, NY: Springer New York. pp. 201–236. ISBN 978-1-4613-8660-5.
- Haynes, R. J. (October 1982). "Effects of liming on phosphate availability in acid soils". Plant and Soil. 68 (3): 289–308. doi:10.1007/BF02197935.
- Ellis, Boyd; Foth, Henry (2017-03-09). Soil Fertility, Second Edition. pp. 73–74. ISBN 9781566702430. Retrieved 2017-05-19.
- "Sodic soils". plantsinaction.science.uq.edu.au. Retrieved 19 May 2017.
- USDA PLANTS Database (2017). "PLANTS Database Advanced Search using minimum and maximum pH". plants.usda.gov. USDA NCRS. Retrieved 2 June 2017.
- Plants for a Future. "Plant Database Search". www.pfaf.org. Retrieved 22 May 2017.
- Hill, M.O.; Mountford, J.O.; Roy, D.B.; Bunce, R.G.H. (1999). Ellenberg's indicator values for British plants. ECOFACT Volume 2. Technical Annex (PDF). Institute of Terrestrial Ecology. ISBN 978-1870393485. Retrieved 29 May 2017.
- Lee, J.A. (1998). "The calcicole-calcifuge problem revisited". Advances in Botanical Research. 29: 13. ISBN 9780080561837. Retrieved 5 June 2017.
- Scott, B.J.; Fisher, J.A. (1989). "Selection of genotypes tolerant of aluminium and manganese". In Robson, A.D. (ed.). Soil acidity and plant growth. Sydney: Academic Press. pp. 167–203. ISBN 978-0125906555. Retrieved 5 June 2017.
- Aitken, R.L.; Moody, P.W.; Mckinley, P.G. (1990). "Lime requirement of acidic Queensland soils. I. Relationships between soil properties and pH buffer capacity". Australian Journal of Soil Research. 28 (5): 695–701. doi:10.1071/SR9900695.
- Von Uexkull, H.R. (1986). "Lime and liming". Efficient Fertilizer Use in Acid Upland Soils of the Humid Tropics. Food & Agriculture Org. pp. 16–22. ISBN 9789251023877. Retrieved 5 June 2017.
- Cox, Loralie. "SOLUTIONS TO SOIL PROBLEMS" (PDF).
- "Soil Quality Indicators: pH" (PDF). NCRS.USDA.
- "Solutions to Soil Problems: High pH – eXtension". Retrieved 2017-02-26.