Scilloideae
Scilloideae (named after the genus Scilla, "squill") is a subfamily of bulbous plants within the family Asparagaceae. Scilloideae is sometimes treated as a separate family Hyacinthaceae, named after the genus Hyacinthus. Scilloideae or Hyacinthaceae include many familiar garden plants such as Hyacinthus (hyacinths), Hyacinthoides (bluebells), Muscari (grape hyacinths) and Scilla and Puschkinia (squills or scillas). Some are important as cut flowers.
| Scilloideae | |
|---|---|
 | |
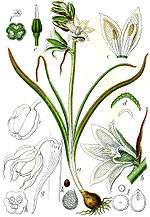
| Scilla bifolia | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Monocots |
| Order: | Asparagales |
| Family: | Asparagaceae |
| Subfamily: | Scilloideae Burnett[1] |
| Genera | |
|
See text | |
| Synonyms[2] | |
| |
Scilloideae are distributed mostly in Mediterranean climates, including South Africa, Central Asia and South America. Their flowers have six tepals and six stamens with a superior ovary, which previously placed them within the lily family (Liliaceae), and their leaves are fleshy, mucilaginous, and arranged in a basal rosette.
The Scilloideae, like most lily-like monocots, were at one time placed in a very broadly defined lily family (Liliaceae). The subfamily is recognized in modern classification systems such as the APG III system of 2009. It is also treated as the separate family Hyacinthaceae, as it is by many researchers and was in earlier APG systems. Determining the boundaries between genera within the Scilloideae is an active area of research. The number of genera varies widely from source to source, from about 30 to about 70. The situation has been described as being in a "state of flux".[3]
Description


The subfamily contains many popular spring-flowering garden bulbs, such as hyacinths (Hyacinthus), grape hyacinths (Muscari), bluebells (Hyacinthoides) and squills (Scilla). Other members are summer- and autumn-flowering, including Galtonia and Eucomis ('pineapple lilies'). Most are native to Mediterranean climate zones and neighboring areas in the Mediterranean Basin and South Africa. Others are found in Central Asia, the Far East and South America.
Morphologically the subfamily is characterised by having 6 tepals and 6 stamens with a superior ovary, a characteristic which placed them within the older order of Liliales in many older classification systems, such as the Cronquist system, but they now separate from them within the Asparagales order. They have also been included in the family Liliaceae.
Roots: contractile and mucilaginous.
Leaves: fleshy and mucilaginous arranged in a basal rosette, alternate and spiral, simple, margin entire, with parallel venation, sheathing at the base, without stipules and hair simple.
Flowers: arranged in scapiflorous inflorescences (in racemes, in spikes, and in heads). The peduncles are articulated. The flowers are hermaphroditic, actinomorphic, often showy.
Perianths: six tepals divided into two whorls, free or joined (connate). When joined, the perianth forms a tubular bell. The tepals are imbricate and petaloid. The corolla may be white, yellow, violet, blue, brown and even black (see images).
Androecium: composed of 6 stamens (exceptionally 3, as in Albuca, for example), with the filaments free or adnate to the tube, often appendiculate. The anthers are dorsifixed and pollen dehiscence occurs by longitudinal openings. The pollen is monosulcate (having a linear furrow).
Gynoecium: superior ovary, tricarpelate, connate and trilocular. Single stigma, capitate to 3-lobed. May contain from one to several ovules in each locule. They have nectaries at the septa of the ovaries.
Fruit: dehiscence loculicidal.
Seed: Seed morphology is diverse, from globular to flattened, and occasionally aril. The seed coat usually contains phytomelan (phytomelanin), one of the defining characteristics of the order, a black pigment present in the seed coat, creating a dark crust.
Chromosomes: Chromosome size varies widely, from 1.2 to 18 µm in length, karyotype bimodal or trimodal. The basic chromosome number is also very variable (X = 2, 6, 7, 10, 15, 17, etc.).[4][5]
Systematics
When treated as a subfamily, the name Scilloideae is derived from the generic name of the type genus, Scilla, and is attributed to Gilbert Thomas Burnett in 1835.[1] When treated as a family, the name Hyacinthaceae is derived from the type genus Hyacinthus, and is usually attributed to August Batsch from ("ex") a 1797 publication by Moritz Borkhausen.[2]
Phylogeny
The monophyly of Scilloideae is well supported by studies based on molecular data.[6] These studies also give support to the exclusion of Camassia, Chlorogalum and related genera, i.e. the former Hyacinthaceae subfamily Chlorogaloideae, now placed in the subfamily Agavoideae.[7][8]
The exact position of the Scilloideae within the broadly defined Asparagaceae is less clear. One possible phylogeny for the seven subfamilies recognised within the family is shown below.[9]
| Asparagaceae |
| ||||||||||||||||||||||||||||||||||||
Although generally agreeing on the main division of the Asparagaceae into two clades, studies have produced slightly different relationships among the Agavoideae, Aphyllanthoideae, Brodiaeoideae and Scilloideae. For example, Seberg et al. (2012) present analyses based on parsimony and on maximum likelihood. In the first, the Scilloideae are sister to the Agavoideae; in the second, they are sister to the Brodiaeoideae.[6]
Early classifications
Detailed historical accounts of taxonomic issues relating to the modern subfamily Scilloideae have been provided by Pfosser & Speta (1999)[10] and Chase et al. (2009).[3] The lilioid monocots have long created classification problems. At one extreme, e.g. in the Cronquist system of 1968, they have been regarded as one large family (Liliaceae sensu lato). At the other extreme, e.g. in the Dahlgren system of 1985, they have been divided between orders and split into many often small families. Dahlgren divided the lilioid monocots in search of monophyly, but in practice he was unsuccessful. His major contribution was to split the Liliaceae into two families, the true Liliaceae, Liliaceae sensu stricto, and the Hyacinthaceae (families which are now placed in separate orders, Liliales and Asparagales).
Splitting off the Hyacinthaceae from the Liliaceae was originally suggested by Batsch in 1786.[11] Batsch's version of the family only superficially resembles the modern version, but did include Hyacinthus and Lachenalia. The group was reduced to a tribe by Endlicher in 1836, and included Camassia. In 1866 Salisbury redistributed the genera into several families.[12] In the 1870s, Baker used tribes to divide up the Liliaceae s.l.. introducing the Hyacintheae, Scilleae, Massonieae, and Chlorogaleae.[13] In 1887 Engler divided the Liliaceae s.l. into two tribes, Lilieaoe and Scilleae.[14] In the twentieth century, Fritsch proposed the division of Liliaceae s.l. into smaller more homogeneous families.[15] In the 1930s the Viennese school elevated Engler's tribes to subfamilies.[16] They questioned the inclusion of such different groups as Lilioideae and Scilloideae within the same family, and even Scilloideae was considered to be composed of at least three groups.[17] By 1969, Huber was recognizing the Scilloideae as the family Hyacinthaceae, and dividing it into tribes.[18] How many tribes were recognised and how the genera were distributed within those tribes depended on the diagnostic characters chosen. Huber used seeds, while Schulze in 1980 used pollen.[19] Morphology and chromosome analysis were supplemented by chemotaxonomy, due to the presence of cardiac steroids, such as the bufadienolids in the Urgineoideae and cardenolids in Ornithogaloideae. Even Linnaean genera such as Hyacinthus, Scilla and Ornithoglum proved heterogeneous and characters useful in other families failed to define satisfactory taxa.
Modern classifications
Modern classification systems for plants are largely derived from molecular phylogenetic analysis. The initial molecular analysis of the Liliaceae s.l. was based on the Dahlgren system, as for example in the work by Chase et al. in 1995.[7] When it was discovered that the Dahlgren families were not monophyletic, the tendency was to create new families out of each identified clade, as in the first Angiosperm Phylogeny Group system of 1998, the APG system. This placed many lilioid families and genera in the order Asparagales (a term derived from Dahlgren, and the largest monocot order). One of the 29 families into which the Asparagales were divided was the Hyacinthaceae.[20]
With further work it was evident that these 29 families, some of which had few genera, could be grouped into larger clades. The APG II system of 2003 was a compromise. It divided the Asparagales into 14 broadly defined families, while allowing an alternative system in which some of the larger families could be replaced by smaller ones. The Hyacinthaceae was one of these optional smaller families, which could alternatively be sunk into a broadly defined Asparagaceae.[21]
This compromise approach was abandoned in the APG III system of 2009, which allowed only the broader families. The paper presenting the system states "The area around Asparagaceae is difficult from the standpoint of circumscription. Although Asparagaceae s.l. are heterogeneous and poorly characterized, Asparagaceae s.s., Agavaceae, Laxmanniaceae, Ruscaceae and even Hyacinthaceae have few if any distinctive features."[22] At the same time, Chase et al. provided subfamilies to replace the alternative narrowly defined families of APG II. The Hyacinthaceae became the subfamily Scilloideae of the family Asparagaceae.[3]
Many sources have adopted the APG III system; for example, the World Checklist of Selected Plant Families places genera such as Hyacinthus only in the broadly defined Asparagaceae.[23] Other sources prefer to retain the narrower families of APG II; for example, Seberg et al. say that it "remains a moot point whether the difficult-to-recognize bracketed families of APG II are a worse or a better choice than the equally difficult-to-recognize subfamilies of APG III", and in their analyses of the phylogeny of the Asparagales they continue to use families such as Hyacinthaceae.[6]
Tribes
In 1990, Pfosser and Speta stated that their earlier classification of the Hyacinthaceae into the subfamilies Hyacinthoideae, Ornithogaloideae, Oziroeoideae and Urgineoideae continued to be supported by ongoing studies. (They further divided the subfamilies Hyacinthoideae and Ornithogaloideae into tribes.)[10] A part of reducing the Hyacinthaceae to the subfamily Scilloideae, Chase et al. (2009) suggested dividing it into four tribes, corresponding to Pfosser and Speta's four subfamilies: Hyacintheae Dumort., Ornithogaleae Rouy, Oziroëeae M.W.Chase, Reveal & M.F.Fay and Urgineeae Rouy.[3] The possible relationship of the four tribes is represented in the following cladogram,[24] which has, however, only "moderate" statistical support.[5]
| Scilloideae |
| |||||||||||||||||||||||||||||||||
The exact boundaries between genera within these tribes remains controversial;[10][24][25] the situation has been described as being in a "state of flux".[3]
Oziroëeae
Species are found only in western South America. They have flowers with stamens which are joined to the petals, rounded seeds and the embryo as long as the seed. The basic chromosome numbers are n = 15, 17. The tribe contains only the genus Oziroë.[5]
Ornithogaleae
In terms of the number of species, this is the largest tribe. Its species are distributed in Europe, western Asia and Africa. They have flowers with three stamens which have flattened filaments. Their seeds are flattened and angular. The basic chromosome numbers range from n = 2 to n = 10.[5] In the treatment by Manning et al. (2009) and Stevens at the Angiosperm Phylogeny Website, the tribe contains four genera, Albuca (about 110–140 species), Dipcadi, Ornithogalum (about 160 species, including Galtonia and Neopatersonia) and Pseudogaltonia.[5][26] By contrast, Martínez-Azorín et al. (2011) divide the tribe into 19 genera.[27]
Urgineeae
Species within this tribe contain bufadienolides and are distributed mainly in Africa, Madagascar, and the Mediterranean through to India. The seeds are flattened and winged with the head barely attached to the endosperm. The basic chromosome numbers are n = 6, 7 and 10.[5] Depending on the source, the tribe may include the genera Bowiea, Drimia (including Urginea), Schizobasis (sometimes included in Drimia) and Fusifilum (also sometimes included in Drimia).[24]
Hyacintheae
In terms of the number of species, this is the second largest tribe. Its species have leaves with pustules or spots, rounded seeds and contain homoisoflavanones. The tribe can in turn be divided into three clades (subtribes):[5]
- Pseudoprospero Speta
- The only species in the genus, Pseudoprospero firmifolium, is from eastern South Africa. It has two ovules per carpel with one seed per locule and a basic chromosome number n = 9.[5]
- Massoniinae Bentham & Hooker f.
- Species are distributed in Africa south of the Sahara and India. There are two or more ovules per carpel. The seeds have elaiosomes. The basic chromosome number is 5 to 10+ (many 20).[5] The subtribe contains about 13–20 genera (depending on the treatment), including Daubenya, Drimiopsis, Eucomis, Lachenalia (about 110 species), Ledebouria (about 80 species), Massonia (including Whiteheadia), Merwilla, Schizocarphus and Veltheimia.[28]
- Hyacinthinae Parlatore
- Species are distributed in Europe, the Mediterranean and North Africa and the Middle East, and then again in the Far East. There are two to eight ovules per carpel; elaiosomes are present in the seeds; and the basic chromosome number is 4 to 8+.[5] The subtribe contains about 14–25 genera (depending on the treatment), including Bellevalia (about 50 species), Brimeura, Hyacinthoides, Muscari (about 50 species), Scilla (about 30 species) and Prospero (about 25 species).[5]
Genera and species
Some genera that were formerly placed within the Scillioideae (as Hyacinthaceae), e.g., Chlorogalum and Camassia, are currently placed in the Agavoideae.[29]
Both historically and as of March 2013, there has been "considerable disagreement over generic limits" in the remaining Scilloideae, with different sources listing from 15 to 45 genera for sub-Saharan Africa alone.[5] The total number of genera has been given as anything between about 30 (with about 500–700 species)[4] and 70 (with about 1000 species).[10]
List of genera
Unless otherwise noted, the list below is based on genera accepted by the World Checklist of Selected Plant Families as in the family Asparagaceae (with synonyms from the same source),[30] with assignments to the subfamily Scilloideae based on the Germplasm Resources Information Network.[31] As noted above, other sources divide up some of these genera, creating a significantly larger number; thus the genus Ornithogalum as conceived by Manning et al. (2009) is divided by Martínez-Azorín et al. (2011) into a more narrowly circumscribed Ornithogalum plus an additional 11 genera.[27]
- Albuca L. (including Battandiera Maire, Coilonox Raf., Stellarioides Medik., Trimelopter Raf.; sometimes included in Ornithogalum[31])
- Alrawia (Wendelbo) Perss. & Wendelbo
- Austronea[32] Martinez-Azorín et al.
- Barnardia Lindl.
- Bellevalia Lapeyr. (including Strangweja Bertol.)
- Bowiea Harv. ex Hook.f. (Climbing Onion, Sea Onion)
- Brimeura Salisb.
- Daubenya Lindl. (including Amphisiphon W.F.Barker, Androsiphon Schltr.)
- Dipcadi Medik. (sometimes included in Ornithogalum[31])
- Drimia Jacq. (including Litanthus Harv., Rhadamanthus Salisb., Rhodocodon Baker, Sypharissa Salisb., Tenicroa Raf., Thuranthos C.H.Wright, Urginea Steinh., Urgineopsis Compton)
- Drimiopsis Lindl. & Paxton (sometimes included in Ledebouria[31])
- Eucomis L'Hér.
- Fessia Speta (sometimes included in Scilla[31])
- Fusifilum Raf. (sometimes included in Drimia[31])
- Hyacinthella Schur
- Hyacinthoides Heist. ex Fabr. (including Endymion Dumort.)
- Hyacinthus Tourn. ex L.
- Lachenalia Jacq. ex Murray (including Brachyscypha Baker, Periboea Kunth, Polyxena Kunth)
- Ledebouria Roth
- Leopoldia Parl. (sometimes included in Muscari[31])
- Massonia Thunb. ex Houtt. (including Neobakeria Schltr., Whiteheadia Harv.)
- Merwilla Speta
- Muscari Mill. (including Botryanthus Kunth, Muscarimia Kostel.)
- Namophila U.Müll.-Doblies & D.Müll.-Doblies[33]
- Ornithogalum L. (including Avonsera Speta, Cathissa Salisb., Eliokarmos Raf., Elsiea F.M.Leight., Ethesia Raf., Galtonia Decne., Honorius Gray, Loncomelos Raf., Melomphis Raf., Neopatersonia Schönland, Nicipe Raf.)
- Oziroe Raf. (including Fortunatia J.F.Macbr.)
- Prospero Salisb.
- Pseudogaltonia (Kuntze) Engl. (sometimes included in Ornithogalum[31])
- Pseudomuscari Garbari & Greuter (sometimes included in Muscari[31])
- Pseudoprospero Speta
- Puschkinia Adams
- Resnova van der Merwe
- Schizobasis Baker (sometimes included in Drimia[31])
- Schizocarphus van der Merwe
- Scilla L. (including Autonoe, Chionodoxa Boiss., Chouardia, Nectaroscilla, Oncostema)
- Spetaea Wetschnig & Pfosser
- Veltheimia Gled.
- Zagrosia Speta (sometimes included in Scilla[31])
Distribution and ecology
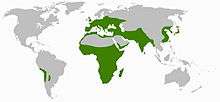
Scilloideae are widely but discontinuously distributed. The genus Oziroe is found only in parts of western South America. Other genera occur in Africa south of the Sahara and parts of the Arabian Peninsula, on both sides of the Mediterranean, further north in Europe through the Middle East to India, and on the east coast of Asia, in China, Korea and Japan. Scilloideae are found in temperate to tropical habitats, but are more diverse in areas of Mediterranean climate (i.e., with a pronounced dry season during the summer).
Scilloideae reproduce both sexually and asexually. The showy flowers of many species of the subfamily are pollinated by a wide range of insects including bees, wasps, flies and moths, as well as birds. Both nectar and pollen act as incentives to pollinating species. Vegetative reproduction may be by bulbils or by seeds through apomixis. The dispersal of seeds may occur by water, wind, or by ants attracted by elaiosomes.
Uses
Cultivation
Many members of the subfamily are popular garden plants, such as Hyacinthus, Muscari, Scilla, Puschkinia, Hyacinthoides, and Ornithogalum (including those formerly placed in Galtonia).
In South Africa the species of Eucomis, Ornithogalum, Veltheimia, among others, are grown as ornamentals. Ornithogalum thyrsoides and the different cultivars of hyacinths are important in the cut flower market.[34]
Medicinal use
Drimia maritima, the sea squill, has been used as a medicinal plant since ancient times. Its use for treatment of edema is mentioned in a papyrus from 1554 BC, the Middle Kingdom of Egypt. Bufadienolides isolated from Drimia maritima and Drimia indica are used for the production of substances for the treatment of heart conditions.
Food
The Scilloideae are only occasionally used as food plants for humans. In Italy the bulbs of Leopoldia comosa are grown for food[35] and in Greece they are consumed as pickles. In France the inflorescence of Ornithogalum pyrenaicum is consumed as a vegetable. In Africa some tribes consume the bulbs of Ledebouria apertiflora and Ledebouria revoluta.
Toxicity
Many Scilloideae produce poisonous steroidal saponins such as bufadienolides and cardenolides, making them inedible.
Several species are toxic. In South Africa, for example, Ornithogalum thyrsoides, and several Ledebouria species (Ledebouria cooperi, L. inguinata, L. ovatifolia, L. revoluta), Ornithogalum saundersiae and several members of the tribe Urgineeae are poisonous to livestock. Scilliroside (a bufadienolide) is used to poison rats, traditionally by spreading dried chips of Drimia maritima bulbs.[36]
References
- Burnett, Gilbert Thomas (1835). Outlines of Botany. London: J. Churchill. OCLC 9537633. p. 428. Cited in Chase, Reveal & Fay 2009, p. 135.
- IPNI Plant Name Query Results for Hyacinthaceae. 1. The International Plant Names Index. Retrieved 2013-03-27.
- Chase, M.W.; Reveal, J.L.; Fay, M.F. (2009). "A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae". Botanical Journal of the Linnean Society. 161 (2): 132–136. doi:10.1111/j.1095-8339.2009.00999.x.CS1 maint: ref=harv (link)
- Watson, L.; Dallwitz, M.J. "Hyacinthaceae". The families of flowering plants. Retrieved 2013-03-23.
- Stevens, P.F. "Angiosperm Phylogeny Website: Asparagales: Scilloideae".
- Seberg, Ole; Petersen, Gitte; Davis, Jerrold I.; Pires, J. Chris; Stevenson, Dennis W.; Chase, Mark W.; Fay, Michael F.; Devey, Dion S.; Jørgensen, Tina; Sytsma, Kenneth J.; Pillon, Yohan (2012). "Phylogeny of the Asparagales based on three plastid and two mitochondrial genes". American Journal of Botany. 99 (5): 875–889. doi:10.3732/ajb.1100468. PMID 22539521.
- Chase, M.W.; Duvall, M. R.; Hills, H.G.; Conran, J.G.; Cox, A.V.; Eguiarte, L.E.; Hartwell, J.; Fay, M.F.; Caddick, L. R.; Cameron, K. M.; Hoot, S. (1995). "Molecular systematics of Lilianae". In Rudall, P.J.; Cribb, P.J.; Cutler, D.F (eds.). Monocotyledons: Systematics and evolution. Royal Botanic Gardens, Kew. pp. 109–137.
- Speta, F. (1998). "Hyacinthaceae". In Kubitzki, K (ed.). The families and genera of vascular plants, vol. 3, Monocotyledons: Lilianae (except Orchidaceae). Berlin: Springer-Verlag.
- Stevens, P.F. "Angiosperm Phylogeny Website: Asparagales".
- Pfosser, Martin; Speta, Franz (1999). "Phylogenetics of Hyacinthaceae based on plastid DNA sequences" (PDF). Annals of the Missouri Botanical Garden. 86 (4): 852–875. doi:10.2307/2666172. JSTOR 2666172.
- Batsch, A.I.G.C. (1786). Dispositio generum plantarum Jenensium secundum Linnaeum et familias naturales (in Latin). Jenae. OCLC 84354311.
- Salisbury, R.A. (1866). The Genera of Plants : A Fragment Containing Part of Liriogamae. London: J. v. Voorst.
- Baker, J.G. (1870). "Monograph of Scilla: Ledebouria and Drimiopsis". Saunder's Ref. Bot. 3. Appendix: 1–18.
Baker, J.G. (1871). "A revision of the genera and species of herbaceous capsular gamophyllous Liliaceae". J. Linn. Soc., Bot. 11 (54–55): 349–436. doi:10.1111/j.1095-8339.1870.tb00068.x. hdl:2027/hvd.32044106471428.
Baker, J.G. (1873). "Revision of the genera and species of Scilleae and Chlorogaleae". J. Linn. Soc., Bot. 13 (68): 209–292. doi:10.1111/j.1095-8339.1872.tb00093.x. - Engler, A. (1887). "Liliaceae". In Engler, A.; Prantl, K (eds.). Die naaturlichen Pflanzenfamilien 11. pp. 10–91.
- Fritsch, K. (1932). "Die systematische Gruppierung der Monokotylen". Ber. Dtsch. Bot. Ges. 50a: 162–184.
- Krause, K. (1930). "Liliaceae". In Engler, A.; Prantl, K (eds.). Die naturlichen Pflanzenfamilien 2 Aufl., 15a. pp. 227–386.
- Buchner, L. (1949). "Vergleichende embryologische Studien an Scilloideae". Österr. Bot. Z. 95 (4): 428–450. doi:10.1007/bf01256694.
- Huber, H. (1969). "Die Samenmerkmale und Verwandtschaftsverhaltnisse der Liliifloren". Mitt. Bot. Staatssamml. Munchen. 8: 219–538.
- Schulze, Werner (1980). "Beiträge zur Taxonomie der Liliifloren: VI Der Umfang der Liliaceae". Wissenschaftliche Zeitschrift der Friedrich-Schiller-Universität Jena, Mathematisch-naturwissenschaftliche Reihe. 29: 607–636.
- The Angiosperm Phylogeny Group (1998). "An ordinal classification for the families of flowering plants". Annals of the Missouri Botanical Garden. 85 (4): 531–553. doi:10.2307/2992015. JSTOR 2992015.
- Angiosperm Phylogeny Group (2003). "An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II" (PDF). Botanical Journal of the Linnean Society. 141 (4): 399–436. doi:10.1046/j.1095-8339.2003.t01-1-00158.x. Archived from the original (PDF) on 2010-12-24.
- Angiosperm Phylogeny Group (2009). "An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III". Botanical Journal of the Linnean Society. 161 (2): 105–121. doi:10.1111/j.1095-8339.2009.00996.x.
- "Hyacinthus". World Checklist of Selected Plant Families. Royal Botanic Gardens, Kew. Retrieved 2013-03-27.
- Manning, J.C.; Goldblatt, P.; Fay, M.F. (2004). "A revised generic synopsis of Hyacintheaceae in sub-Saharan Africa, based on molecular evidence, including new combinations and the new tribe Pseudoprospereae". Edinburgh Journal of Botany. 60 (3): 533–568. doi:10.1017/S0960428603000404.
- Wetschnig, W.; Pfosser, M. (2003). "The Scilla plumbea puzzle – present status of the genus Scilla sensu lato in southern Africa and description of Spetaea lachenaliiflora, a new genus and species of Massonieae (Hyacinthaceae)". Taxon. 52 (1): 75–91. doi:10.2307/3647303. JSTOR 3647303.
- Manning, John C.; Forest, Félix; Devey, Dion S.; Fay, Michael F.; Goldblatt, Peter (2009). "A molecular phylogeny and a revised classification of Ornithogaloideae (Hyacinthaceae) based on an analysis of four plastid DNA regions". Taxon. 58 (1): 77–107. doi:10.1002/tax.581011. JSTOR 27756826.
- Martinez-Azorin, Mario; Crespo, Manuel B.; Juan, Ana; Fay, Michael F. (2011). "Molecular phylogenetics of subfamily Ornithogaloideae (Hyacinthaceae) based on nuclear and plastid DNA regions, including a new taxonomic arrangement". Annals of Botany. 107 (1): 1–37. doi:10.1093/aob/mcq207. PMC 3002468. PMID 21163815.
- Pfosser, M.; Wetschnig, W.; Ungar, S.; Prenner, G. (2004). "Phylogenetic relationships among genera of Massonieae (Hyacinthaceae) inferred from plastid DNA and seed morphology". Journal of Plant Research. 116 (2): 115–132. doi:10.1007/s10265-003-0076-8. PMID 12736783.
- Stevens, P.F. "Angiosperm Phylogeny Website: Asparagales: Agavoideae".
- WCSP (2011). "search for "Asparagaceae"". World Checklist of Selected Plant Families. The Board of Trustees of the Royal Botanic Gardens, Kew. Retrieved 2013-03-22.
- "Query GRIN Taxonomy for Plants". Germplasm Resources Information Network. United States Department of Agriculture. Retrieved 2013-03-22.
- Martínez-Azorín, Mario; Crespo, Manuel B.; Alonso-Vargas, María Ángeles; Dold, Anthony P.; Pinter, Michael; Wetschnig, Wolfgang (2018). "Austronea (Asparagaceae, Scilloideae), a new genus from southern Africa, including the description of seven new species". Phytotaxa. 365 (2): 101–129. doi:10.11646/phytotaxa.365.2.1. hdl:10045/78227.
- "Namophila U.Müll.-Doblies & D.Müll.-Doblies". eMonocot. Retrieved 2013-03-22.
- Pfosser, Martin; Speta, Franz. "Hyacinthaceae". Tree of Life Web Project. Retrieved 2013-03-23.
- http://www.lampascione.it/ Lampascioni.it (in Italian)
- el Bahri, L.; Djegham, M.; Makhlouf, M. (2000). "Urginea maritima L. (Squill): A Poisonous Plant of North Africa". Veterinary and Human Toxicology. 42 (2): 108–110. PMID 10750179.
Bibliography
- Mabberley, D. (1997). The Plant-Book. Cambridge, United Kingdom: Cambridge University Press.
- Judd, W.S.; Campbell, C.S.; Kellogg, E.A.; Stevens, P.F.; Donoghue, M.J. (2007). "Hyacinthaceae". Plant Systematics: A Phylogenetic Approach (3rd ed.). Sunderland, Massachusetts: Sinauer Associates. pp. 269–270. ISBN 978-0-87893-407-2.
- Stedje, Brita (2001). "Generic Delimitation of Hyacinthaceae, with Special Emphasis on Sub-Saharan Genera". Systematics and Geography of Plants. 71 (2): 449–454. doi:10.2307/3668693. JSTOR 3668693.
External links
| Wikimedia Commons has media related to Scilloideae. |