Sirtuin 1
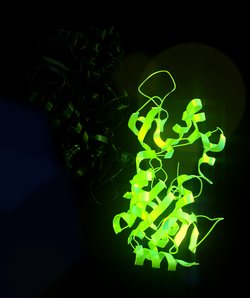
Sirtuin 1, also known as NAD-dependent deacetylase sirtuin-1, is a protein that in humans is encoded by the SIRT1 gene.[5][6][7]
SIRT1 stands for sirtuin (silent mating type information regulation 2 homolog) 1 (S. cerevisiae), referring to the fact that its sirtuin homolog (biological equivalent across species) in yeast (S. cerevisiae) is Sir2. SIRT1 is an enzyme that deacetylates proteins that contribute to cellular regulation (reaction to stressors, longevity).[8]
Function
Sirtuin 1 is a member of the sirtuin family of proteins, homologs of the Sir2 gene in S. cerevisiae. Members of the sirtuin family are characterized by a sirtuin core domain and grouped into four classes. The functions of human sirtuins have not yet been determined; however, yeast sirtuin proteins are known to regulate epigenetic gene silencing and suppress recombination of rDNA. Studies suggest that the human sirtuins may function as intracellular regulatory proteins with mono-ADP-ribosyltransferase activity. The protein encoded by this gene is included in class I of the sirtuin family.[6]
Sirtuin 1 is downregulated in cells that have high insulin resistance and inducing its expression increases insulin sensitivity, suggesting the molecule is associated with improving insulin sensitivity.[9] Furthermore, SIRT1 was shown to de-acetylate and affect the activity of both members of the PGC1-alpha/ERR-alpha complex, which are essential metabolic regulatory transcription factors.[10][11][12][13][14][15]
In mammals, SIRT1 has been shown to deacetylate and thereby deactivate the p53 protein.[16] SIRT1 also stimulates autophagy by preventing acetylation of proteins (via deacetylation) required for autophagy as demonstrated in cultured cells and embryonic and neonatal tissues. This function provides a link between sirtuin expression and the cellular response to limited nutrients due to caloric restriction.[17]
Human aging is characterized by a chronic, low-grade inflammation level,[18] and NF-κB is the main transcriptional regulator of genes related to inflammation.[19] SIRT1 inhibits NF-κB-regulated gene expression by deacetylating the RelA/p65 subunit of NF-κB at lysine 310.[20][21]
SIRT1 plays a role in activating T helper 17 cells, which contribute to autoimmune disease; efforts to activate SIRT1 therapeutically may trigger or exacerbate autoimmune disease.[22]
SIRT1, along with HDAC1 and the AP-1 promoter complex within D1-type dopaminergic medium spiny neurons, appears to be closely involved in the pathogenesis of addiction.
Selective ligands
Activators
- Lamin A is a protein that had been identified as a direct activator of Sirtuin 1 during a study on progeria.[23]
- Resveratrol has been claimed to be an activator of Sirtuin 1,[24] but this effect has been disputed based on the fact that the initially used activity assay, using a non-physiological substrate peptide, can produce artificial results.[25][26] Resveratrol increases the expression of SIRT1, meaning that it does increase the activity of SIRT1, though not necessarily by direct activation.[9] However, resveratrol was later shown to directly activate Sirtuin 1 against non-modified peptide substrates.[27][28] Resveratrol also enhances the binding between Sirtuin 1 and Lamin A.[23] In addition to resveratrol, a range of other plant-derived polyphenols have also been shown to interact with SIRT1.[29]
- SRT-1720 was also claimed to be an activator,[24] but this now has been questioned.[30]
- Methylene blue[31] by increasing NAD+/NADH ratio.
- Metformin activates both PRKA and SIRT1.[32]
Interactions
Sirtuin 1 has been shown to interact with HEY2,[33] PGC1-alpha,[12] ERR-alpha,[10] and AIRE.[34] Mir-132 microRNA has been reported to interact with Sirtuin 1 mRNA, so as to reduce protein expression. This has been linked to insulin resistance in the obese.[35]
Human Sirt1 has been reported having 136 direct interactions in interactomic studies involved in numerous processes.[36]
Sir2
Sir2 (whose homolog in mammals is known as SIRT1) was the first gene of the sirtuin genes to be found. It was found in budding yeast, and, since then, members of this highly conserved family have been found in nearly all organisms studied.[37] Sirtuins are hypothesized to play a key role in an organism's response to stresses (such as heat or starvation) and to be responsible for the lifespan-extending effects of calorie restriction.[38][39]
The three letter yeast gene symbol Sir stands for Silent Information Regulator while the number 2 is representative of the fact that it was the second SIR gene discovered and characterized.[40][41]
In the roundworm, Caenorhabditis elegans, Sir-2.1 is used to denote the gene product most similar to yeast Sir2 in structure and activity.[42][43]
Method of action and observed effects
Sirtuins act primarily by removing acetyl groups from lysine residues within proteins in the presence of NAD+; thus, they are classified as "NAD+-dependent deacetylases" and have EC number 3.5.1.[44] They add the acetyl group from the protein to the ADP-ribose component of NAD+ to form O-acetyl-ADP-ribose. The HDAC activity of Sir2 results in tighter packaging of chromatin and a reduction in transcription at the targeted gene locus. The silencing activity of Sir2 is most prominent at telomeric sequences, the hidden MAT loci (HM loci), and the ribosomal DNA (rDNA) locus (RDN1) from which ribosomal RNA is transcribed.
Limited overexpression of the Sir2 gene results in a lifespan extension of about 30%,[45] if the lifespan is measured as the number of cell divisions the mother cell can undergo before cell death. Concordantly, deletion of Sir2 results in a 50% reduction in lifespan.[45] In particular, the silencing activity of Sir2, in complex with Sir3 and Sir4, at the HM loci prevents simultaneous expression of both mating factors which can cause sterility and shortened lifespan.[46] Additionally, Sir2 activity at the rDNA locus is correlated with a decrease in the formation of rDNA circles. Chromatin silencing, as a result of Sir2 activity, reduces homologous recombination between rDNA repeats, which is the process leading to the formation of rDNA circles. As accumulation of these rDNA circles is the primary way in which yeast are believed to "age", then the action of Sir2 in preventing accumulation of these rDNA circles is a necessary factor in yeast longevity.[46]
Starving of yeast cells leads to a similarly extended lifespan, and indeed starving increases the available amount of NAD+ and reduces nicotinamide, both of which have the potential to increase the activity of Sir2. Furthermore, removing the Sir2 gene eliminates the life-extending effect of caloric restriction.[47] Experiments in the nematode Caenorhabditis elegans and in the fruit fly Drosophila melanogaster[48] support these findings. As of 2006, experiments in mice are underway.[38]
However, some other findings call the above interpretation into question. If one measures the lifespan of a yeast cell as the amount of time it can live in a non-dividing stage, then silencing the Sir2 gene actually increases lifespan [49] Furthermore, calorie restriction can substantially prolong reproductive lifespan in yeast even in the absence of Sir2.[50]
In organisms more complicated than yeast, it appears that Sir2 acts by deacetylation of several other proteins besides histones.
Resveratrol is a substance that has been shown through experiment to have a number of life-extending and health benefits in various species; it also increases the activity of Sir2, which is the postulated reason for its beneficial effects. Resveratrol is produced by plants when they are stressed, and it is possible that plants use the substance to increase their own Sir2 activity in order to survive periods of stress.[38] Although there is mounting evidence for this hypothesis, its validity is debated.[30][25][51][26]
In the fruit fly Drosophilia melanogaster, the Sir2 gene does not seem to be essential; loss of a sirtuin gene has only very subtle effects.[47] However, mice lacking the SIRT1 gene (the sir2 biological equivalent) were smaller than normal at birth, often died early or became sterile.[52]
Activation of SIRT1
Increased expression of SIRT1 protein, when induced by a synthetic small molecule activator of SIRT1 (SRT2104), extended both the mean and maximal lifespan of mice.[53] Another SIRT1 activator (SRT1720) also extended lifespan of mice.[54]
Homologous recombination
SIRT1 protein actively promotes homologous recombination (HR) in human cells, and likely promotes recombinational repair of DNA breaks.[55] SIRT1 mediated HR requires the WRN protein.[55] WRN protein functions in double-strand break repair by HR.[56] WRN protein is a RecQ helicase, and in its mutated form gives rise to Werner syndrome, a genetic condition in humans characterized by numerous features of premature aging. These findings link SIRT1 function to HR, a DNA repair process that is likely necessary for maintaining the integrity of the genome during aging.[55]
References
- GRCh38: Ensembl release 89: ENSG00000096717 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000020063 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Frye RA (June 1999). "Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity". Biochemical and Biophysical Research Communications. 260 (1): 273–79. doi:10.1006/bbrc.1999.0897. PMID 10381378.
- "Entrez Gene: SIRT1 sirtuin (silent mating type information regulation 2 homolog) 1 (S. cerevisiae)".
- SIRT1 human gene location in the UCSC Genome Browser.
- Sinclair DA, Guarente L (March 2006). "Unlocking the Secrets of Longevity Genes". Scientific American. 294 (3): 48–51, 54–7. Bibcode:2006SciAm.294c..48S. doi:10.1038/scientificamerican0306-48. PMID 16502611.
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q (October 2007). "SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B". Cell Metabolism. 6 (4): 307–19. doi:10.1016/j.cmet.2007.08.014. PMID 17908559.
- Wilson BJ, Tremblay AM, Deblois G, Sylvain-Drolet G, Giguère V (July 2010). "An acetylation switch modulates the transcriptional activity of estrogen-related receptor alpha". Molecular Endocrinology. 24 (7): 1349–58. doi:10.1210/me.2009-0441. PMC 5417470. PMID 20484414.
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (March 2005). "Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1". Nature. 434 (7029): 113–8. Bibcode:2005Natur.434..113R. doi:10.1038/nature03354. PMID 15744310.
- Nemoto S, Fergusson MM, Finkel T (April 2005). "SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}". The Journal of Biological Chemistry. 280 (16): 16456–60. doi:10.1074/jbc.M501485200. PMID 15716268.
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J (December 2006). "Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha". Cell. 127 (6): 1109–22. doi:10.1016/j.cell.2006.11.013. PMID 17112576.
- Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J, Olefsky J, Guarente L, Montminy M (November 2008). "A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange". Nature. 456 (7219): 269–73. Bibcode:2008Natur.456..269L. doi:10.1038/nature07349. PMC 2597669. PMID 18849969.
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J (April 2009). "AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity". Nature. 458 (7241): 1056–60. Bibcode:2009Natur.458.1056C. doi:10.1038/nature07813. PMC 3616311. PMID 19262508.
- EntrezGene 23411 Human Sirt1
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T (March 2008). "A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy". Proceedings of the National Academy of Sciences of the United States of America. 105 (9): 3374–89. Bibcode:2008PNAS..105.3374L. doi:10.1073/pnas.0712145105. PMC 2265142. PMID 18296641.
- Franceschi C, Campisi J (June 2014). "Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 69 (S1): S4–S9. doi:10.1093/gerona/glu057. PMID 24833586.
- Lawrence T (December 2009). "The nuclear factor NF-kappaB pathway in inflammation". Cold Spring Harbor Perspectives in Biology. 1 (6): a001651. doi:10.1101/cshperspect.a001651. PMC 2882124. PMID 20457564.
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW (June 2004). "Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase". The EMBO Journal. 23 (12): 2369–80. doi:10.1038/sj.emboj.7600244. PMC 423286. PMID 15152190.
- Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A (October 2013). "Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders". Cellular Signalling. 25 (10): 1939–48. doi:10.1016/j.cellsig.2013.06.007. PMID 23770291.
- Verdin, E (4 December 2015). "NAD⁺ in aging, metabolism, and neurodegeneration". Science. 350 (6265): 1208–13. Bibcode:2015Sci...350.1208V. doi:10.1126/science.aac4854. PMID 26785480.
- Liu B, Ghosh S, Yang X, Zheng H, Liu X, Wang Z, Jin G, Zheng B, Kennedy BK, Suh Y, Kaeberlein M, Tryggvason K, Zhou Z (December 2012). "Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria". Cell Metabolism. 16 (6): 738–50. doi:10.1016/j.cmet.2012.11.007. PMID 23217256.
- Alcaín FJ, Villalba JM (April 2009). "Sirtuin activators". Expert Opinion on Therapeutic Patents. 19 (4): 403–14. doi:10.1517/13543770902762893. PMID 19441923.
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK (April 2005). "Substrate-specific activation of sirtuins by resveratrol". The Journal of Biological Chemistry. 280 (17): 17038–45. doi:10.1074/jbc.M500655200. PMID 15684413.
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M (December 2009). "Resveratrol is not a direct activator of SIRT1 enzyme activity". Chemical Biology & Drug Design. 74 (6): 619–24. doi:10.1111/j.1747-0285.2009.00901.x. PMID 19843076.
- Lakshminarasimhan M, Rauh D, Schutkowski M, Steegborn C (March 2013). "Sirt1 activation by resveratrol is substrate sequence-selective". Aging. 5 (3): 151–54. doi:10.18632/aging.100542. PMC 3629287. PMID 23524286.
- Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA (March 2013). "Evidence for a common mechanism of SIRT1 regulation by allosteric activators". Science. 339 (6124): 1216–19. Bibcode:2013Sci...339.1216H. doi:10.1126/science.1231097. PMC 3799917. PMID 23471411.
- Ajami M, Pazoki-Toroudi H, Amani H, Nabavi SF, Braidy N, Vacca RA, Atanasov AG, Mocan A, Nabavi SM (November 2016). "Therapeutic role of sirtuins in neurodegenerative disease and their modulation by polyphenols". Neuroscience and Biobehavioral Reviews. 73: 39–47. doi:10.1016/j.neubiorev.2016.11.022. PMID 27914941.
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K (March 2010). "SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1". The Journal of Biological Chemistry. 285 (11): 8340–51. doi:10.1074/jbc.M109.088682. PMC 2832984. PMID 20061378.
- Shin SY, Kim TH, Wu H, Choi YH, Kim SG (March 2014). "SIRT1 activation by methylene blue, a repurposed drug, leads to AMPK-mediated inhibition of steatosis and steatohepatitis". European Journal of Pharmacology. 727: 115–24. doi:10.1016/j.ejphar.2014.01.035. PMID 24486702.
- Song YM, Lee YH, Kim JW, Ham DS, Kang ES, Cha BS, Lee HC, Lee BW (2015). "Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway". Autophagy. 11 (1): 46–59. doi:10.4161/15548627.2014.984271. PMC 4502778. PMID 25484077.
- Takata T, Ishikawa F (January 2003). "Human Sir2-related protein SIRT1 associates with the bHLH repressors HES1 and HEY2 and is involved in HES1- and HEY2-mediated transcriptional repression". Biochemical and Biophysical Research Communications. 301 (1): 250–57. doi:10.1016/S0006-291X(02)03020-6. PMID 12535671.
- Chuprin A, Avin A, Goldfarb Y, Herzig Y, Levi B, Jacob A, Sela A, Katz S, Grossman M, Guyon C, Rathaus M, Cohen HY, Sagi I, Giraud M, McBurney MW, Husebye ES, Abramson J (July 2015). "The deacetylase Sirt1 is an essential regulator of Aire-mediated induction of central immunological tolerance". Nature Immunology. 16 (7): 737–45. doi:10.1038/ni.3194. PMID 26006015.
- Strum JC, Johnson JH, Ward J, Xie H, Feild J, Hester A, Alford A, Waters KM (November 2009). "MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1". Molecular Endocrinology. 23 (11): 1876–84. doi:10.1210/me.2009-0117. PMC 5419165. PMID 19819989.
- Sharma A, Gautam V, Costantini S, Paladino A, Colonna G (2012). "Interactomic and pharmacological insights on human sirt-1". Frontiers in Pharmacology. 3: 40. doi:10.3389/fphar.2012.00040. PMC 3311038. PMID 22470339.
- Frye RA (July 2000). "Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins". Biochemical and Biophysical Research Communications. 273 (2): 793–98. doi:10.1006/bbrc.2000.3000. PMID 10873683.
- Sinclair DA, Guarente L (March 2006). "Unlocking the secrets of longevity genes". Scientific American. 294 (3): 48–51, 54–57. Bibcode:2006SciAm.294c..48S. doi:10.1038/scientificamerican0306-48. PMID 16502611.
- Noriega LG, Feige JN, Canto C, Yamamoto H, Yu J, Herman MA, Mataki C, Kahn BB, Auwerx J (September 2011). "CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability". EMBO Reports. 12 (10): 1069–76. doi:10.1038/embor.2011.151. PMC 3185337. PMID 21836635.
- Rine J, Herskowitz I (May 1987). "Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae". Genetics. 116 (1): 9–22. PMC 1203125. PMID 3297920.
- North BJ, Verdin E (2004). "Sirtuins: Sir2-related NAD-dependent protein deacetylases". Genome Biology. 5 (5): 224. doi:10.1186/gb-2004-5-5-224. PMC 416462. PMID 15128440.
- WormBase Protein Summary: Sir-2.1
- http://mediwire.skyscape.com/main/Default.aspx?P=Content&ArticleID=174239 Archived September 27, 2007, at the Wayback Machine Skyscape Content: Do antiaging approaches promote longevity?
- The Sir2 protein family from EMBL's InterPro database
- Chang KT, Min KT (June 2002). "Regulation of lifespan by histone deacetylase". Ageing Research Reviews. 1 (3): 313–26. doi:10.1016/S1568-1637(02)00003-X. PMID 12067588.
- Kaeberlein M, McVey M, Guarente L (October 1999). "The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms". Genes & Development. 13 (19): 2570–80. doi:10.1101/gad.13.19.2570. PMC 317077. PMID 10521401.
- EntrezGene 34708 Drosophilia Sir2
- Rogina B, Helfand SL (November 2004). "Sir2 mediates longevity in the fly through a pathway related to calorie restriction". Proceedings of the National Academy of Sciences of the United States of America. 101 (45): 15998–6003. Bibcode:2004PNAS..10115998R. doi:10.1073/pnas.0404184101. PMC 528752. PMID 15520384.
- Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD (November 2005). "Sir2 blocks extreme life-span extension". Cell. 123 (4): 655–67. doi:10.1016/j.cell.2005.08.042. PMID 16286010.
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK (September 2004). "Sir2-independent life span extension by calorie restriction in yeast". PLoS Biology. 2 (9): E296. doi:10.1371/journal.pbio.0020296. PMC 514491. PMID 15328540.
- Borra MT, Smith BC, Denu JM (April 2005). "Mechanism of human SIRT1 activation by resveratrol". The Journal of Biological Chemistry. 280 (17): 17187–95. doi:10.1074/jbc.M501250200. PMID 15749705.
- McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M (January 2003). "The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis". Molecular and Cellular Biology. 23 (1): 38–54. doi:10.1128/MCB.23.1.38-54.2003. PMC 140671. PMID 12482959.
- Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang Y, Becker KG, Khraiwesh H, González-Reyes JA, Villalba JM, Baur JA, Elliott P, Westphal C, Vlasuk GP, Ellis JL, Sinclair DA, Bernier M, de Cabo R (2014). "SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass". Aging Cell. 13 (5): 787–96. doi:10.1111/acel.12220. PMC 4172519. PMID 24931715.
- Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M, de Cabo R (2014). "The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet". Cell Rep. 6 (5): 836–43. doi:10.1016/j.celrep.2014.01.031. PMC 4010117. PMID 24582957.
- Uhl M, Csernok A, Aydin S, Kreienberg R, Wiesmüller L, Gatz SA (2010). "Role of SIRT1 in homologous recombination". DNA Repair (Amst.). 9 (4): 383–93. doi:10.1016/j.dnarep.2009.12.020. PMID 20097625.
- Thompson LH, Schild D (2002). "Recombinational DNA repair and human disease" (PDF). Mutat. Res. 509 (1–2): 49–78. doi:10.1016/s0027-5107(02)00224-5. PMID 12427531.
Further reading
- Frye RA (June 1999). "Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity". Biochemical and Biophysical Research Communications. 260 (1): 273–79. doi:10.1006/bbrc.1999.0897. PMID 10381378.
- Frye RA (July 2000). "Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins". Biochemical and Biophysical Research Communications. 273 (2): 793–98. doi:10.1006/bbrc.2000.3000. PMID 10873683.
- Wiemann S, Weil B, Wellenreuther R, Gassenhuber J, Glassl S, Ansorge W, Böcher M, Blöcker H, Bauersachs S, Blum H, Lauber J, Düsterhöft A, Beyer A, Köhrer K, Strack N, Mewes HW, Ottenwälder B, Obermaier B, Tampe J, Heubner D, Wambutt R, Korn B, Klein M, Poustka A (March 2001). "Toward a catalog of human genes and proteins: sequencing and analysis of 500 novel complete protein coding human cDNAs". Genome Research. 11 (3): 422–35. doi:10.1101/gr.GR1547R. PMC 311072. PMID 11230166.
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W (October 2001). "Negative control of p53 by Sir2alpha promotes cell survival under stress". Cell. 107 (2): 137–48. doi:10.1016/S0092-8674(01)00524-4. PMID 11672522.
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA (October 2001). "hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase". Cell. 107 (2): 149–59. doi:10.1016/S0092-8674(01)00527-X. PMID 11672523.
- Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T (May 2002). "Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence". The EMBO Journal. 21 (10): 2383–96. doi:10.1093/emboj/21.10.2383. PMC 126010. PMID 12006491.
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA (November 2002). "Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1". The Journal of Biological Chemistry. 277 (47): 45099–107. doi:10.1074/jbc.M205670200. PMID 12297502.
- Travers H, Spotswood HT, Moss PA, Turner BM (November 2002). "Human CD34+ hematopoietic progenitor cells hyperacetylate core histones in response to sodium butyrate, but not trichostatin A". Experimental Cell Research. 280 (2): 149–58. doi:10.1006/excr.2002.5632. PMID 12413881.
- Takata T, Ishikawa F (January 2003). "Human Sir2-related protein SIRT1 associates with the bHLH repressors HES1 and HEY2 and is involved in HES1- and HEY2-mediated transcriptional repression". Biochemical and Biophysical Research Communications. 301 (1): 250–57. doi:10.1016/S0006-291X(02)03020-6. PMID 12535671.
- Senawong T, Peterson VJ, Avram D, Shepherd DM, Frye RA, Minucci S, Leid M (October 2003). "Involvement of the histone deacetylase SIRT1 in chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2-mediated transcriptional repression". The Journal of Biological Chemistry. 278 (44): 43041–50. doi:10.1074/jbc.M307477200. PMC 2819354. PMID 12930829.
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA (September 2003). "Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan". Nature. 425 (6954): 191–96. Bibcode:2003Natur.425..191H. doi:10.1038/nature01960. PMID 12939617.
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME (March 2004). "Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase". Science. 303 (5666): 2011–15. Bibcode:2004Sci...303.2011B. doi:10.1126/science.1094637. PMID 14976264.
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L (February 2004). "Mammalian SIRT1 represses forkhead transcription factors". Cell. 116 (4): 551–63. doi:10.1016/S0092-8674(04)00126-6. PMID 14980222.
- van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM (July 2004). "FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1)". The Journal of Biological Chemistry. 279 (28): 28873–79. doi:10.1074/jbc.M401138200. PMID 15126506.
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW (June 2004). "Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase". The EMBO Journal. 23 (12): 2369–80. doi:10.1038/sj.emboj.7600244. PMC 423286. PMID 15152190.
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L (June 2004). "Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma". Nature. 429 (6993): 771–76. Bibcode:2004Natur.429..771P. doi:10.1038/nature02583. PMC 2820247. PMID 15175761.
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA (July 2004). "Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase". Science. 305 (5682): 390–92. Bibcode:2004Sci...305..390C. doi:10.1126/science.1099196. PMID 15205477.
- Miyazaki S, Kakutani K, Yurube T, Maeno K, Takada T, Zhang Z, Kurakawa T, Terashima Y, Ito M, Ueha T, Matsushita T, Kuroda R, Kurosaka M, Nishida K (September 2015). "Recombinant human SIRT1 protects against nutrient deprivation-induced mitochondrial apoptosis through autophagy induction in human intervertebral disc nucleus pulposus cells". Arthritis Research & Therapy. 17: 253. doi:10.1186/s13075-015-0763-6. PMC 4571076. PMID 26373839.
External links
- Corante weblog by Derek Lowe about sir2 and SIRT1 research.
- SIRT1 human gene location in the UCSC Genome Browser.
- SIRT1 human gene details in the UCSC Genome Browser.