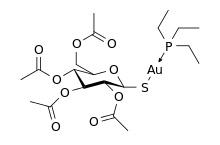
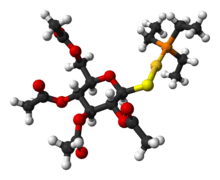
Auranofin
Auranofin is a gold salt classified by the World Health Organization as an antirheumatic agent. It has the brand name Ridaura.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ridaura |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a685038 |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 40%[1][2] |
| Protein binding | 60%[1][2] |
| Metabolism | Plasma membrane of the cell removes the acetyl groups of the glucose moiety. |
| Elimination half-life | 21-31 hours[1][2] |
| Excretion | Urine (60%), faeces[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.077 |
| Chemical and physical data | |
| Formula | C20H34AuO9PS0 |
| Molar mass | 678.48 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Use
Auranofin is used to treat rheumatoid arthritis. It improves arthritis symptoms including painful or tender and swollen joints and morning stiffness.[3] Auranofin is a safer treatment compared to the more common injectable gold thiolates (gold sodium thiomalate and gold thioglucose), but meta-analysis of 66 clinical trials concluded that it is somewhat less effective.[4]
The drug was approved for the treatment of rheumatoid arthritis in 1985. No longer a first-line treatment for rheumatoid arthritis, due to "its adverse effects, most of which are associated with long-term use for chronic disease. The most common adverse effects are gastrointestinal complaints such as loose stools, abdominal cramping and watery diarrhoea, which can develop in the early months of treatment. The development of loose stools occurs in 40 % of patients, while watery diarrhoea is reported in just 2–5 % of patients, and in most cases these symptoms were alleviated by reducing or splitting the dose".[5]
Research
HIV infection
Auranofin is under investigation as a means of reducing the viral reservoir of HIV that lies latent in the body's T-cells despite treatment with antiretroviral therapy.[6] The drug was shown to reduce the amount of latent virus in monkey trials.[7] A human study testing auranofin and other investigational treatments is ongoing in Brazil.[8] Preliminary results show that auranofin contributed to a decrease in the viral reservoir.[9]
Amebiasis
Auranofin has been identified in a high-throughput drug screen as 10 times more potent than metronidazole against Entamoeba histolytica, the protozoan agent of human amebiasis. Assays of thioredoxin reductase and transcriptional profiling suggest that the effect of auranofin on the enzyme enhances the sensitivity of the trophozoites to reactive oxygen-mediated killing in mouse and hamster models; the results are marked reductions of the number of parasites, the inflammatory reaction to the infestation, and the damage to the liver.[10][11][12]
Tuberculosis
In a cell-based screen, auranofin showed potent activity against replicating and non-replicating M. tuberculosis as well as other gram-positive bacteria. Auranofin protected mice from an otherwise lethal infection with methicillin-resistant S. aureus (MRSA). The drug acts in a similar manner in bacteria as in parasites by inhibiting thioredoxin reductase (TrxR). Studies in humans are needed to evaluate the potential of this drug to treat Gram-positive bacterial infections in humans.[13]
Ovarian cancer
Drug-screening reveals auranofin induces apoptosis in ovarian cancer cells in vitro.[14][15]
COVID-19
Auranofin may inhibit replication of SARS-CoV-2, the virus responsible for causing COVID-19 in cell culture. Inflammation may also be reduced.[16]
References
- Kean, WF; Hart, L; Buchanan, WW (May 1997). "Auranofin" (PDF). British Journal of Rheumatology. 36 (5): 560–72. doi:10.1093/rheumatology/36.5.560. PMID 9189058.
- "Ridaura (auranofin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 13 March 2014.
- MedlinePlus DrugInfo medmaster-a685038
- Felson, David T.; Anderson, Jennifer J.; Meenan, Robert F. (October 1990). "The comparative efficacy and toxicity of second-line drugs in rheumatoid arthritis results of two metaanalyses". Arthritis & Rheumatism. 33 (10): 1449–1461. doi:10.1002/art.1780331001. PMID 1977391.
- Roder, Christine; Thomson, Melanie J. (2015). "Auranofin: Repurposing an Old Drug for a Golden New Age". Drugs in R&D volume. 15: 13–20. doi:10.1007/s40268-015-0083-y.
- Gold-based drug shows promise in clearing HIV reservoir in monkey study. Keith Alcorn. AIDSmaps.com. Accessed 23 April 2011.
- Lewis, Mark G.; DaFonseca, Sandrina; Chomont, Nicolas; Palamara, Anna T.; Tardugno, Maria; Mai, Antonello; Collins, Matt; Wagner, Wendeline L.; Yalley-Ogunro, Jake; Greenhouse, Jack; Chirullo, Barbara; Norelli, Sandro; Garaci, Enrico; Savarino, Andrea (2011-07-17). "Gold drug auranofin restricts the viral reservoir in the monkey AIDS model and induces containment of viral load following ART suspension". AIDS. 25 (11): 1347–56. doi:10.1097/QAD.0b013e328347bd77. ISSN 0269-9370. PMID 21505294.
- "Multi Interventional Study Exploring HIV-1 Residual Replication: a Step Towards HIV-1 Eradication and Sterilizing Cure - Full Text View - ClinicalTrials.gov". Retrieved 2018-08-14.
- "Auranofin plus nicotinamide impact HIV reservoir among ART suppressed HIV individuals". Retrieved 2018-08-16.
- Debnath, Anjan; Parsonage, Derek; Andrade, Rosa M; He, Chen; Cobo, Eduardo R; Hirata, Ken; Chen, Steven; García-Rivera, Guillermina; Orozco, Esther; Martínez, Máximo B; Gunatilleke, Shamila S; Barrios, Amy M; Arkin, Michelle R; Poole, Leslie B; McKerrow, James H; Reed, Sharon L (2012). "A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target". Nature Medicine. 18 (6): 956–60. doi:10.1038/nm.2758. PMC 3411919. PMID 22610278.
- "Drug Found for Parasite That Is Major Cause of Death Worldwide". Science Daily.
- "Arthritis Drug Effective Against Global Parasite, Study Suggests". Science Daily.
- Harbut, Michael B; Vilcheze, Catherine; Luo, Xiaozhou; Hensler, Mary E; Guo, Hui; Yang, Baiyuan; Chatterjee, Arnab K; Nizet, Victor; Jacobs Jr., William R; Schultz, Peter G; Wang, Feng (2015). "Auranofin exerts broad-spectrum bactericidal activities by targeting thio-redox homeostasis". PNAS. 112 (14): 4453–4458. doi:10.1073/pnas.1504022112. PMC 4394260. PMID 25831516.
- Park, SH; Lee, JH; Berek, JS; Hu, MC (2014). "Auranofin displays anticancer activity against ovarian cancer cells through FOXO3 activation independent of p53". Int J Oncol. 45 (4): 1691–8. doi:10.3892/ijo.2014.2579. PMC 4151813. PMID 25096914.
- Oommen, Deepu; Yiannakis, Dennis; Jha, Awadhesh N. (2016). "BRCA1 deficiency increases the sensitivity of ovarian cancer cells to auranofin". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 784-785: 8–15. doi:10.1016/j.mrfmmm.2015.11.002. PMID 26731315.
- "Georgia State Researchers Find Rheumatoid Arthritis Drug Is Effective Against Coronavirus". News Hub. 15 April 2020. Retrieved 15 April 2020.
Further reading
- Jeon K, Byun M, Jue D (2003). "Gold compound auranofin inhibits IkappaB kinase (IKK) by modifying Cys-179 of IKKbeta subunit". Experimental & Molecular Medicine. 35 (2): 61–6. doi:10.1038/emm.2003.9. PMID 12754408.
- Kim I, Jin J, Lee I, Park S (2004). "Auranofin induces apoptosis and when combined with retinoic acid enhances differentiation of acute promyelocytic leukaemia cells in vitro". Br J Pharmacol. 142 (4): 749–55. doi:10.1038/sj.bjp.0705708. PMC 1575039. PMID 15159275.
- Venardos K, Harrison G, Headrick J, Perkins A (2004). "Auranofin increases apoptosis and ischaemia-reperfusion injury in the rat isolated heart". Clin Exp Pharmacol Physiol. 31 (5–6): 289–94. doi:10.1111/j.1440-1681.2004.03993.x. PMID 15191400.
- Hafejee A, Winhoven S, Coulson I (2004). "Jessner's lymphocytic infiltrate responding to oral auranofin". J Dermatolog Treat. 15 (5): 331–2. doi:10.1080/09546630410016924. PMID 15370403.
- Rigobello M, Folda A, Baldoin M, Scutari G, Bindoli A (2005). "Effect of auranofin on the mitochondrial generation of hydrogen peroxide. Role of thioredoxin reductase". Free Radic Res. 39 (7): 687–95. doi:10.1080/10715760500135391. PMID 16036347.
- Suarez-Almazor ME, Spooner CH, Belseck E, Shea B (2000). Suarez-Almazor ME (ed.). "Auranofin versus placebo in rheumatoid arthritis". Cochrane Database Syst Rev (2): CD002048. doi:10.1002/14651858.CD002048. PMID 10796461.