Tetrabromoauric acid
Tetrabromoauric acid is an inorganic compound with the formula HAuBr4. It is the bromide analog of chloroauric acid. It is generated analogously, by reacting a mixture of hydrobromic and nitric acids with elemental gold.[1][2]
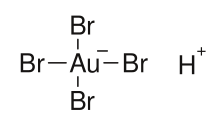 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ECHA InfoCard | 100.037.385 |
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| AuBr4H | |
| Molar mass | 517.591 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Weick, C. F.; Basolo, Fred (1966). "The Aqueous Solution Chemistry and Kinetic Behavior of a Pseudo-Octahedral Complex of Gold(III)". Inorg. Chem. 5 (4): 576. doi:10.1021/ic50038a018.
- Afanasieva, V. A.; Glinskaya, L. A.; Klevtsova, R. F.; Mironov, I. V.; Sheludyakova, L. A. (2007). "Introduction of halogen atoms into gold(III) tetraaza metallocomplexes. Crystal and molecular structure of [Au(C9H18N4Br)](ClO4)2 and [Au(C14H20N4Br2)]ClO4". J. Struc. Chem. 48 (2): 289. doi:10.1007/s10947-007-0045-5.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.