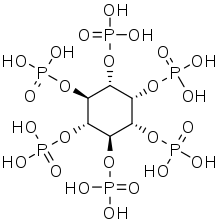
Phytic acid
Phytic acid is a six-fold dihydrogenphosphate ester of inositol (specifically, of the myo isomer), also called inositol hexakisphosphate (IP6) or inositol polyphosphate. At physiological pH, the phosphates are partially ionized, resulting in the phytate anion.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(1R,2S,3r,4R,5S,6s)-cyclohexane-1,2,3,4,5,6-hexayl hexakis[dihydrogen (phosphate)] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.369 | ||
| E number | E391 (antioxidants, ...) | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H18O24P6 | |||
| Molar mass | 660.029 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
The (myo) phytate anion is a colorless species that has significant nutritional role as the principal storage form of phosphorus in many plant tissues, especially bran and seeds. It is also present in many legumes, cereals, and grains. Phytic acid and phytate have a strong binding affinity to the dietary minerals, calcium, iron, and zinc, inhibiting their absorption.[1]
The lower inositol polyphosphates are inositol esters with less than six phosphates, such as inositol penta- (IP5), tetra- (IP4), and triphosphate (IP3). These occur in nature as catabolites of phytic acid.
Significance in agriculture
Phosphorus and inositol in phytate form are not, in general, bioavailable to non-ruminant animals because these animals lack the digestive enzyme phytase required to hydrolyze the inositol-phosphate linkages. Ruminants are readily able to digest phytate because of the phytase produced by rumen microorganisms.[2]
In most commercial agriculture, non-ruminant livestock, such as swine, fowl, and fish,[3] are fed mainly grains, such as maize, legumes, and soybeans.[4] Because phytate from these grains and beans is unavailable for absorption, the unabsorbed phytate passes through the gastrointestinal tract, elevating the amount of phosphorus in the manure.[2] Excess phosphorus excretion can lead to environmental problems, such as eutrophication.[5] The use of sprouted grains will reduce the quantity of phytic acids in feed, with no significant reduction of nutritional value.[6]
Also, viable low-phytic acid mutant lines have been developed in several crop species in which the seeds have drastically reduced levels of phytic acid and concomitant increases in inorganic phosphorus.[7] However, germination problems have reportedly hindered the use of these cultivars thus far. This may be due to phytic acid's critical role in both phosphorus and metal ion storage.[8] Phytate variants also have the potential to be used in soil remediation, to immobilize uranium, nickel and other inorganic contaminants.[9]
Biological and physiological roles
Although indigestible for many animals, phytic acid and its metabolites as they occur in seeds and grains have several important roles for the seedling plant.
Most notably, phytic acid functions as a phosphorus store, as an energy store, as a source of cations and as a source of myo-inositol (a cell wall precursor). Phytic acid is the principal storage form of phosphorus in plant seeds.[10]
In animal cells, myo-inositol polyphosphates are ubiquitous, and phytic acid (myo-inositol hexakisphosphate) is the most abundant, with its concentration ranging from 10 to 100 μM in mammalian cells, depending on cell type and developmental stage.[11][12]
This compound is not obtained from the animal diet, but must be synthesized inside the cell from phosphate and inositol (which in turn is produced from glucose, usually in the kidneys). The interaction of intracellular phytic acid with specific intracellular proteins has been investigated in vitro, and these interactions have been found to result in the inhibition or potentiation of the physiological activities of those proteins.[13][14] The best evidence from these studies suggests an intracellular role for phytic acid as a cofactor in DNA repair by nonhomologous end-joining.[13] Other studies using yeast mutants have also suggested intracellular phytic acid may be involved in mRNA export from the nucleus to the cytosol.[15][16]
Inositol hexaphosphate facilitates the formation of the six-helix bundle and assembly of the immature HIV-1 Gag lattice. IP6 makes ionic contacts with two rings of lysine residues at the centre of the Gag hexamer. Proteolytic cleavage then unmasks an alternative binding site, where IP6 interaction promotes the assembly of the mature capsid lattice. These studies identify IP6 as a naturally occurring small molecule that promotes both assembly and maturation of HIV-1.[17]
Food science
Phytic acid was discovered in 1903.[18] Phytic acid, mostly as phytate in the form of phytin, is found within the hulls of seeds, including nuts, grains and pulses.[1] In-home food preparation techniques can break down the phytic acid in all of these foods. Simply cooking the food will reduce the phytic acid to some degree. More effective methods are soaking in an acid medium, sprouting and lactic acid fermentation such as in sourdough and pickling.[19] No detectable phytate (less than 0.02% of wet weight) was observed in vegetables such as scallion and cabbage leaves or in fruits such as apples, oranges, bananas, or pears.[20]
As a food additive, phytic acid is used as the preservative, E391.[21][22]
Dry food sources of phytic acid[23][20][24][25][26][27][28][29] Food Proportion by weight (g/100 g) Min. Max. Pumpkin seed 4.3 4.3 Linseed 2.15 2.78 Sesame seeds flour 5.36 5.36 Chia seeds 0.96 1.16 Almonds 1.35 3.22 Brazil nuts 1.97 6.34 Coconut 0.36 0.36 Hazelnut 0.65 0.65 Peanut 0.95 1.76 Walnut 0.98 0.98 Maize (corn) 0.75 2.22 Oat 0.42 1.16 Oat meal 0.89 2.40 Brown rice 0.84 0.99 Polished rice 0.14 0.60 Wheat 0.39 1.35 Wheat flour 0.25 1.37 Wheat germ 0.08 1.14 Whole wheat bread 0.43 1.05 Beans, pinto 2.38 2.38 Buckwheat 1.00 1.00 Chickpeas 0.56 0.56 Lentils 0.44 0.50 Soybeans 1.00 2.22 Tofu 1.46 2.90 Soy beverage 1.24 1.24 Soy protein concentrate 1.24 2.17 New potato 0.18 0.34 Spinach 0.22 NR Avocado fruit 0.51 0.51 Chestnuts[30] 0.47
Fresh food sources of phytic acid[25] Food Proportion by weight (%) Min. Max. Taro 0.143 0.195 Cassava 0.114 0.152
Dietary mineral absorption
Phytic acid has a strong binding affinity to the dietary minerals, calcium, iron, and zinc, inhibiting their absorption.[1][31] Phytochemicals like polyphenols and tannins also influence the binding.[32] When iron and zinc bind to phytic acid, they form insoluble precipitates and are far less absorbable in the intestines. This process can therefore contribute to iron and zinc deficiencies in people whose diets rely on these foods for their mineral intake, such as those in developing countries[33][34] and vegetarians.[35]
Human nutrition
Because phytic acid can affect the absorption of iron, "dephytinization should be considered as a major strategy to improve iron nutrition during the weaning period".[36] Dephytinization by exogenous phytase to phytate-containing food is an approach being investigated to improve nutritional health in populations that are vulnerable to mineral deficiency due to their reliance on phytate-laden food staples. Crop breeding to increase mineral density (biofortification) or reducing phytate content are under preliminary research.[37]
See also
| Wikimedia Commons has media related to Phytic acid. |
- Antinutrient
- Essential nutrient
- Oxalic acid
References
- Schlemmer, U.; Frølich, W.; Prieto, R. M.; Grases, F. (2009). "Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis" (PDF). Molecular Nutrition & Food Research. 53 Suppl 2: S330–75. doi:10.1002/mnfr.200900099. PMID 19774556.
- Klopfenstein TJ, Angel R, Cromwell G, Erickson GE, Fox DG, Parsons C, Satter LD, Sutton AL, Baker DH (July 2002). "Animal Diet Modification to Decrease the Potential for Nitrogen and Phosphorus Pollution". Council for Agricultural Science and Technology. 21.
- Romarheim OH, Zhang C, Penn M, Liu YJ, Tian LX, Skrede A, Krogdahl Å, Storebakken T (2008). "Growth and intestinal morphology in cobia (Rachycentron canadum) fed extruded diets with two types of soybean meal partly replacing fish meal". Aquaculture Nutrition. 14 (2): 174–180. doi:10.1111/j.1365-2095.2007.00517.x.
- Jezierny, D.; Mosenthin, R.; Weiss, E. (2010-05-01). "The use of grain legumes as a protein source in pig nutrition: A review". Animal Feed Science and Technology - ANIM FEED SCI TECH. 157: 111–128. doi:10.1016/j.anifeedsci.2010.03.001.
- Mallin MA (2003). "Industrialized Animal Production—A Major Source of Nutrient and Microbial Pollution to Aquatic Ecosystems". Population and Environment. 24 (5): 369–385. doi:10.1023/A:1023690824045. JSTOR 27503850.
- Malleshi, N. G.; Desikachar, H. S. R. (1986). "Nutritive value of malted millet flours". Plant Foods for Human Nutrition. 36 (3): 191–6. doi:10.1007/BF01092036.
- Guttieri MJ, Peterson KM, Souza EJ (2006). "Milling and Baking Quality of Low Phytic Acid Wheat". Crop Science. 46 (6): 2403–8. doi:10.2135/cropsci2006.03.0137.
- Shitan, Nobukazu; Yazaki, Kazufumi (2013-01-01), Jeon, Kwang W. (ed.), "Chapter Nine - New Insights into the Transport Mechanisms in Plant Vacuoles", International Review of Cell and Molecular Biology, Academic Press, 305, pp. 383–433, retrieved 2020-04-24
- Seaman JC, Hutchison JM, Jackson BP, Vulava VM (2003). "In situ treatment of metals in contaminated soils with phytate". Journal of Environmental Quality. 32 (1): 153–61. doi:10.2134/jeq2003.0153. PMID 12549554.
- Reddy NR, Sathe SK, Salunkhe DK (1982). Phytates in legumes and cereals. Advances in Food Research. 28. pp. 1–92. doi:10.1016/s0065-2628(08)60110-x. ISBN 9780120164288. PMID 6299067.
- Szwergold BS, Graham RA, Brown TR (December 1987). "Observation of inositol pentakis- and hexakis-phosphates in mammalian tissues by 31P NMR". Biochemical and Biophysical Research Communications. 149 (3): 874–81. doi:10.1016/0006-291X(87)90489-X. PMID 3426614.
- Sasakawa N, Sharif M, Hanley MR (July 1995). "Metabolism and biological activities of inositol pentakisphosphate and inositol hexakisphosphate". Biochemical Pharmacology. 50 (2): 137–46. doi:10.1016/0006-2952(95)00059-9. PMID 7543266.
- Hanakahi LA, Bartlet-Jones M, Chappell C, Pappin D, West SC (September 2000). "Binding of inositol phosphate to DNA-PK and stimulation of double-strand break repair". Cell. 102 (6): 721–9. doi:10.1016/S0092-8674(00)00061-1. PMID 11030616.
- Norris FA, Ungewickell E, Majerus PW (January 1995). "Inositol hexakisphosphate binds to clathrin assembly protein 3 (AP-3/AP180) and inhibits clathrin cage assembly in vitro". The Journal of Biological Chemistry. 270 (1): 214–7. doi:10.1074/jbc.270.1.214. PMID 7814377.
- York JD, Odom AR, Murphy R, Ives EB, Wente SR (July 1999). "A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export". Science. 285 (5424): 96–100. doi:10.1126/science.285.5424.96. PMID 10390371.
- Shears SB (March 2001). "Assessing the omnipotence of inositol hexakisphosphate". Cellular Signalling (Submitted manuscript). 13 (3): 151–8. doi:10.1016/S0898-6568(01)00129-2. PMID 11282453.
- Dick RA, Zadrozny KK, Xu C, Schur FK, Lyddon TD, Ricana CL, Wagner JM, Perilla JR, Ganser-Pornillos BK, Johnson MC, Pornillos O, Vogt VM (August 2018). "Inositol phosphates are assembly co-factors for HIV-1". Nature. 560 (7719): 509–512. doi:10.1038/s41586-018-0396-4. PMC 6242333. PMID 30069050.
- Mullaney EJ, Ullah, Abul H.J. "Phytases: attributes, catalytic mechanisms, and applications" (PDF). United States Department of Agriculture–Agricultural Research Service. Archived from the original (PDF) on 2012-11-07. Retrieved May 18, 2012.
- "Phytates in cereals and legumes". fao.org.
- Phillippy BQ, Wyatt CJ (May 2001). "Degradation of phytate in foods by phytases in fruit and vegetable extracts". Journal of Food Science. 66 (4): 535–539.
- Functional Food - Improve Health through Adequate Food edited by María Chávarri Hueda, pg. 86
- https://noshly.com/additive/391/preservative/391/
- Dephytinisation with Intrinsic Wheat Phytase and Iron Fortification Significantly Increase Iron Absorption from Fonio (Digitaria exilis) Meals in West African Women (2013)
- Reddy NR, Sathe SK (2001). Food Phytates. Boca Raton: CRC. ISBN 978-1-56676-867-2.
- Phillippy BQ, Bland JM, Evens TJ (January 2003). "Ion chromatography of phytate in roots and tubers". Journal of Agricultural and Food Chemistry. 51 (2): 350–3. doi:10.1021/jf025827m. PMID 12517094.
- Macfarlane BJ, Bezwoda WR, Bothwell TH, Baynes RD, Bothwell JE, MacPhail AP, Lamparelli RD, Mayet F (February 1988). "Inhibitory effect of nuts on iron absorption". The American Journal of Clinical Nutrition. 47 (2): 270–4. doi:10.1093/ajcn/47.2.270. PMID 3341259.
- Gordon DT, Chao LS (March 1984). "Relationship of components in wheat bran and spinach to iron bioavailability in the anemic rat". The Journal of Nutrition. 114 (3): 526–35. doi:10.1093/jn/114.3.526. PMID 6321704.
- Arendt EK, Zannini E (2013-04-09). "Chapter 11: Buckwheat". Cereal grains for the food and beverage industries. Woodhead Publishing. p. 388. ISBN 978-0-85709-892-4.
- Pereira Da Silva B. Concentration of nutrients and bioactive compounds in chia (Salvia Hispanica L.), protein quality and iron bioavailability in wistar rats (Ph.D. thesis). Federal University of Viçosa.
- Scuhlz M. "Paleo Diet Guide: With Recipes in 30 Minutes or Less: Diabetes Heart Disease: Paleo Diet Friendly: Dairy Gluten Nut Soy Free Cookbook". PWPH Publications – via Google Books.
- Gupta, R. K.; Gangoliya, S. S.; Singh, N. K. (2013). "Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains". Journal of Food Science and Technology. 52 (2): 676–684. doi:10.1007/s13197-013-0978-y. PMC 4325021. PMID 25694676.
- Prom-u-thai C, Huang L, Glahn RP, Welch RM, Fukai S, Rerkasem B (2006). "Iron (Fe) bioavailability and the distribution of anti-Fe nutrition biochemicals in the unpolished, polished grain and bran fraction of five rice genotypes". Journal of the Science of Food and Agriculture. 86 (8): 1209–15. doi:10.1002/jsfa.2471.
- Hurrell RF (September 2003). "Influence of vegetable protein sources on trace element and mineral bioavailability". The Journal of Nutrition. 133 (9): 2973S–7S. doi:10.1093/jn/133.9.2973S. PMID 12949395.
- Committee on Food Protection; Food and Nutrition Board; National Research Council (1973). "Phytates". Toxicants Occurring Naturally in Foods. National Academy of Sciences. pp. 363–371. ISBN 978-0-309-02117-3.
- American Dietetic Association (June 2003). "Position of the American Dietetic Association and Dietitians of Canada: Vegetarian diets". Journal of the American Dietetic Association. 103 (6): 748–65. doi:10.1053/jada.2003.50142. PMID 12778049.
- Hurrell RF, Reddy MB, Juillerat MA, Cook JD (May 2003). "Degradation of phytic acid in cereal porridges improves iron absorption by human subjects". The American Journal of Clinical Nutrition. 77 (5): 1213–9. CiteSeerX 10.1.1.333.4941. doi:10.1093/ajcn/77.5.1213. PMID 12716674.
- Raboy, Victor (22 January 2020). "Low phytic acid crops: Observations based on four decades of research". Plants. 9 (2): 140. doi:10.3390/plants9020140. ISSN 2223-7747. PMID 31979164.

