Phosphine imide
In chemistry a phosphine imide (sometimes abbreviated to phosphinimide) also known as a iminophosphorane is a functional group with the formula R3P=NR. While structurally related to phosphine oxide its chemistry has more in common with phosphonium ylides.
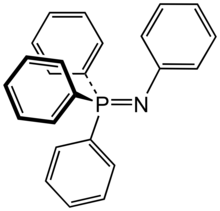
Anions of this group, with the structure R3P=N−, are called phosphinoimidates and are used as ligands to form phosphinimide complexes which are highly active catalysts in some olefin polymerization reactions.[1]
Synthesis
Phosphine imides can be isolated as intermediates in the Staudinger reaction and have also been prepared by the action of hydroxylamine-O-sulfonic acid on phosphines, proceeding via a p-aminophosphonium salt.[2]
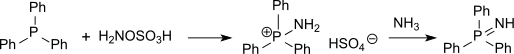
Reactions and applications
The functional group will readily hydrolyse to give a phosphine oxide and an amine
- R3P=NR' + H2O → R3P=O + R'NH2
Phosphinimide ligands of the general formula NPR3− form transition metal phosphinimide complexeses. Some of these complexes are potential catalysts for the synthesis of polyethylene.[1]
See also
References
- Dehnicke, Kurt; Krieger, Matthias; Massa, Werner (February 1999). "Phosphoraneiminato complexes of transition metals". Coordination Chemistry Reviews. 182 (1): 19–65. doi:10.1016/S0010-8545(98)00191-X.
- Appel, Rolf; Büchner, Werner; Guth, Egbert (26 November 1958). "Zur Kenntnis des Imins, I. Über Phosphinimine und Sulfimine". Justus Liebigs Annalen der Chemie. 618 (1): 53–58. doi:10.1002/jlac.19586180107.