Phosphinimide ligands
Phosphinimide ligands, also known as phosphorane iminato ligands, are any of a class of organic compounds of the general formula NPR3−. The R groups represent organic substituents or, in rare cases, halides or NR2 groups. NPR3− is isoelectronic with phosphine oxides (OPR3) and siloxides ([OSiR3]−), but far more basic.[1][2] By varying the R groups on P, a variety of ligands with different electronic and steric properties can be produced, and due to the high oxidation state of phosphorus, these ligands have good thermal stability.[3] Many transition metal phosphinimide complexes have been well-developed as have main group phosphinimide complexes.[1]
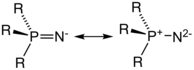
In main group phosphinimide complexes, only terminal and μ2-N-bridging bonding modes are observed.[2] The terminally bound bent ligands are primarily commonly have M-N-P bond angles ranging from 120-150°.[2] Both the M-N and N-P bond lengths are appropriate for double bonds. This bonding can best be described by a covalent single bond with an overlaying share of polar bonding. The μ2-N-bridging mode arises when the free electron pair at nitrogen gives rise to dimerization.[2] These dimeric complexes yield different M-N bond lengths depending on the ligands present in the rest of the ligand sphere of M.[2] When the complex contains two or four identical ligands, nearly equal M-N distances are observed, whereas, when different or odd-numbered identical ligands are in the complex, the M-N distances are all of significantly different length.[2]
Synthesis
Phosphonimines with the formula R3P=NSiMe3 are particularly useful. They are prepared by the Staudinger reaction of tertiary phosphines with trimethylsilyl azide:
- R3P + N3SiMe3 → R3P=NSiMe3 + N2
R3P=NSiMe3 undergoes alcoholysis to give the parent imine:
- R3P=NSiMe3 + MeOH → R3P=NH + MeOSiMe3
Ammonia can be used in place of alcohol.[5][2]
Lithium phosphinimides are produced by deprotonation of the parent imine:
- R3P=NH + RLi → R3P=NLi + RH
The lithio derivatives, which exist as tetrameric clusters in the solid state, are useful reagents.[5][2][6]
References
- Dehnicke, Kurt; Krieger, M.; Massa, W. (1999). "Phosphoraneiminato Complexes of Transition metals". Coordination Chemistry Reviews. 182: 19–65. doi:10.1016/S0010-8545(97)90055-2.
- Dehnicke, Kurt; Weller, F. (1997). "Phosphorane Iminato Complexes of Main Group Elements". Coordination Chemistry Reviews. 158 (1): 103–169. doi:10.1016/S0010-8545(96)01257-X.
- Stephan, Douglas (2006). "Sterically Demanding Phosphinimides: Ligands for Unique Main Group and Transition Metal Chemistry". Advances in Organometallic Chemistry. 54: 267–291. doi:10.1016/S0065-3055(05)54006-1. ISBN 9780120311545.
- Holthausen, Michael H.; Mallov, Ian; Stephan, Douglas W. (2014). "Phosphinimine-substituted boranes and borenium ions". Dalton Trans. 43 (40): 15201–15211. doi:10.1039/C4DT02406K. PMID 25184519.
- Stephan, Douglas W. (2005). "The Road to Early-Transition-Metal Phosphinimide Olefin Polymerization Catalysts". Organometallics. 24 (11): 2548–2560. doi:10.1021/om050096b.
- Courtenay, Silke; Wei, P.; Stephan, D. (2003). "The Syntheses and Structures of Lithium Phosphinimide and Phosphinimine Complexes". Can. J. Chem. 81 (12): 1471–1476. doi:10.1139/V03-162.