Noble metal
In chemistry, the noble metals are metals that are resistant to corrosion and oxidation in moist air (unlike most base metals). The short list of chemically noble metals (those elements upon which almost all chemists agree) comprises ruthenium (Ru), rhodium (Rh), palladium (Pd), silver (Ag), osmium (Os), iridium (Ir), platinum (Pt), and gold (Au).[1]
More inclusive lists include one or more of mercury (Hg),[2][3][4] rhenium (Re),[5] and copper (Cu) as noble metals. On the other hand, titanium (Ti), niobium (Nb), and tantalum (Ta) are not included as noble metals although they are very resistant to corrosion.
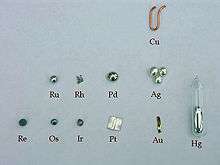
While the noble metals tend to be valuable – due to both their rarity in the Earth's crust and their applications in areas like metallurgy, high technology, and ornamentation (jewelry, art, sacred objects, etc.) – the terms noble metal and precious metal are not synonymous.
The term noble metal can be traced back to at least the late 14th century[6] and has slightly different meanings in different fields of study and application. Only in atomic physics is there a strict definition, which includes only copper, silver, and gold, because they have completely filled d-subshells. For this reason, there are many quite different lists of "noble metals".
In addition to this term's function as a compound noun, there are circumstances where noble is used as an adjective for the noun metal. A galvanic series is a hierarchy of metals (or other electrically conductive materials, including composites and semimetals) that runs from noble to active, and allows one to predict how materials will interact in the environment used to generate the series. In this sense of the word, graphite is more noble than silver and the relative nobility of many materials is highly dependent upon context, as for aluminium and stainless steel in conditions of varying pH.[7]
Properties
Platinum, gold and mercury can be dissolved in aqua regia, a highly concentrated mixture of hydrochloric acid and nitric acid, but iridium cannot. The solubility of silver is limited by the formation of silver chloride precipitate.[8] Palladium and silver are, however, soluble in nitric acid. Ruthenium can be dissolved in aqua regia only when in the presence of oxygen, while rhodium must be in a fine pulverized form. Niobium and tantalum are resistant to all acids, including aqua regia.[9]
Physics
In physics, the definition of a noble metal is most strict. It requires that the d-bands of the electronic structure be filled. From this perspective, only copper, silver and gold are noble metals, as all d-like bands are filled and do not cross the Fermi level.[10] However, d-hybridized bands do cross the Fermi level to a small extent. In the case of platinum, two d bands cross the Fermi level, changing its chemical behaviour such that it can function as a catalyst. The difference in reactivity can easily be seen during the preparation of clean metal surfaces in an ultra-high vacuum: surfaces of "physically defined" noble metals (e.g., gold) are easy to clean and keep clean for a long time, while those of platinum or palladium, for example, are covered by carbon monoxide very quickly.[11]
Predictions
The superheavy elements from hassium to livermorium inclusive are expected to be "partially very noble metals"; chemical investigations of hassium has established that it behaves like its lighter congener osmium, and preliminary investigations of nihonium and flerovium have suggested but not definitively established noble behavior.[12] Copernicium's behaviour seems to partly resemble both its lighter congener mercury and the noble gas radon.[13]
See also
References
- Brooks, Robert R., ed. (1992). Noble Metals and Biological Systems: Their Role in Medicine, Mineral Exploration, and the Environment. Boca Raton, Fla.: CRC Press. ISBN 9780849361647. OCLC 24379749.
- Notes
- A. Holleman, N. Wiberg, "Lehrbuch der Anorganischen Chemie", de Gruyter, 1985, 33. edition, p. 1486
- "Edelmetall". www.uni-protokolle.de. Retrieved April 6, 2018.
- "Dictionary of Mining, Mineral, and Related Terms", Compiled by the American Geological Institute, 2nd edition, 1997
- Scoullos, M.J., Vonkeman, G.H., Thornton, I., Makuch, Z., "Mercury – Cadmium – Lead: Handbook for Sustainable Heavy Metals Policy and Regulation",Series: Environment & Policy, Vol. 31, Springer-Verlag, 2002
- The New Encyclopædia Britannica, 15th edition, Vol. VII, 1976
- "the definition of noble metal". Dictionary.com. Retrieved April 6, 2018.
- Everett Collier, "The Boatowner’s Guide to Corrosion", International Marine Publishing, 2001, p. 21
- W. Xing, M. Lee, Geosys. Eng. 20, 216, 2017
- A. Holleman, N. Wiberg, "Inorganic Chemistry", Academic Press, 2001
- Hüger, E.; Osuch, K. (2005). "Making a noble metal of Pd". EPL. 71 (2): 276. Bibcode:2005EL.....71..276H. doi:10.1209/epl/i2005-10075-5.
- S. Fuchs, T.Hahn, H.G. Lintz, "The oxidation of carbon monoxide by oxygen over platinum, palladium and rhodium catalysts from 10−10 to 1 bar", Chemical engineering and processing, 1994, V 33(5), pp. 363–369
- Nagame, Yuichiro; Kratz, Jens Volker; Matthias, Schädel (December 2015). "Chemical studies of elements with Z ≥ 104 in liquid phase". Nuclear Physics A. 944: 614–639. Bibcode:2015NuPhA.944..614N. doi:10.1016/j.nuclphysa.2015.07.013.
- Mewes, J.-M.; Smits, O. R.; Kresse, G.; Schwerdtfeger, P. (2019). "Copernicium is a Relativistic Noble Liquid". Angewandte Chemie International Edition. doi:10.1002/anie.201906966.
External links
- noble metal – chemistry Encyclopædia Britannica, online edition
- To see which bands cross the Fermi level, the Fermi surfaces of almost all the metals can be found at the Fermi Surface Database
- The following article might also clarify the correlation between band structure and the term noble metal: Hüger, E.; Osuch, K. (2005). "Making a noble metal of Pd". EPL. 71 (2): 276. Bibcode:2005EL.....71..276H. doi:10.1209/epl/i2005-10075-5.