Alternative periodic tables
Alternative periodic tables are tabulations of chemical elements differing in their organization from the traditional depiction of the periodic system.[1][2]
| Part of a series on the |
| Periodic table |
|---|
|
Periodic table forms |
|
By periodic table structure
|
|
|
By other characteristics
|
|
|
|
|
Data pages for elements
|
|
Over a thousand have been devised, often for didactic reasons, as not all correlations between the chemical elements are effectively captured by the standard periodic table.
Major alternative structures
Left-step periodic table (Janet, 1928)
Charles Janet's left-step periodic table[3] is the most widely used alternative to the traditional depiction of the periodic system. It organizes elements according to an idealized orbital filling (instead of valence).[4] For example, the elements Sc to Zn are shown as a 3d block implying orbital occupancy [Ar] 4s2 3dx, although it is now known that Cr actually has orbital occupancy [Ar] 4s1 3d5 and Cu has [Ar] 4s1 3d10.
| f1 | f2 | f3 | f4 | f5 | f6 | f7 | f8 | f9 | f10 | f11 | f12 | f13 | f14 | d1 | d2 | d3 | d4 | d5 | d6 | d7 | d8 | d9 | d10 | p1 | p2 | p3 | p4 | p5 | p6 | s1 | s2 | |
| 1s | H | He | ||||||||||||||||||||||||||||||
| 2s | Li | Be | ||||||||||||||||||||||||||||||
| 2p 3s | B | C | N | O | F | Ne | Na | Mg | ||||||||||||||||||||||||
| 3p 4s | Al | Si | P | S | Cl | Ar | K | Ca | ||||||||||||||||||||||||
| 3d 4p 5s | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | Rb | Sr | ||||||||||||||
| 4d 5p 6s | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | Cs | Ba | ||||||||||||||
| 4f 5d 6p 7s | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | Fr | Ra |
| 5f 6d 7p 8s | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | 119 | 120 |
| f-block | d-block | p-block | s-block | |||||||||||||||||||||||||||||
.svg.png)
Compared to the common layout, the left-step table has these changes:
- Helium is placed in group 2 (not in group 18).
- Groups 1 and 2 (the s-block), including elements 119 and 120 in extended period 8, are moved to the right side of the table.
- The s-block is shifted upwards one row, and all elements not in the s-block are now one row lower than in the standard table. For example, most of the fourth row in the standard table is the fifth row in this table.
In the result, the order is still consistently by atomic number (Z), 1–120.
Two-dimensional spiral (Benfey, 1964)
In Theodor Benfey's periodic table the elements form a two-dimensional spiral, starting from hydrogen, and folding their way around two peninsulas, the transition metals, and lanthanides and actinides. A superactinide island is already slotted in.[5]
Three-dimensional, flower-like (Paul Giguère, 1966)
Paul Giguère's 3-D periodic table consists of four connected billboards with the elements written on the front and the back. The first billboard has the group 1 elements on the front and the group 2 elements at the back, with hydrogen and helium omitted altogether. At a 90° angle the second billboard contains the groups 13 to 18 front and back. Two more billboards each making 90° angles contain the other elements.[6][7]
Three-dimensional, physicist's (Timothy Stowe, 1986)
Timothy Stowe's physicist's periodic table is three-dimensional with the three axes representing the principal quantum number, orbital quantum number, and orbital magnetic quantum number.[8][9] Helium is again a group 2 element.
Elements repeating (Ronald L. Rich, 2005)
Ronald L. Rich has proposed a periodic table where elements appear more than once when appropriate.[10] He notes that hydrogen shares properties with group 1 elements based on valency, with group 17 elements because hydrogen is a non-metal but also with the carbon group based on similarities in chemical bonding to transition metals and a similar electronegativity. In this rendition of the periodic table carbon and silicon also appear in the same group as titanium and zirconium.
ADOMAH (Valery Tsimmerman, 2006)
.svg.png)
The ADOMAH table is an adaptation of the left step table.[12] Each strictly vertical column of the table has the same value of the principal quantum number n. For example, n = 3 for Fe. Each block of elements has the same value of the secondary quantum number l. For example, l = 2 for Fe. Each element entry together with all preceding elements corresponds to the electron configuration of that element (with 20 exceptions out of 118 known elements). For example, the electron configuration of Fe is determined by starting at H, which is 1s1, and counting in atomic number order. This gives a configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d6 or, in short form, [Ar] 4s2 3d6.
The four blocks of the Adomah table can be rearranged such that they fit, equidistantly spaced, inside a regular tetrahedron. The latter, in turn, fits into a cube.[13]
Other
A chemists' table ("Newlands Revisited") with an alternative positioning of hydrogen, helium and the lanthanides was published by EG Marks and JA Marks in 2010.[14]
Variants of the classical layout
From Mendeleev's original periodic table, elements have been basically arranged by valence (groups in columns) and the repetition therein (periods in rows). Over the years and with discoveries in atomic structure, this schema has been adjusted and expanded, but not changed as a principle.

The oldest periodic table is the short form table (columns I–VIII) by Dmitri Mendeleev, which shows secondary chemical kinships. For example, the alkali metals and the coinage metals (copper, silver, gold) are in the same column because both groups tend to have a valence of one. This format is still used by many, as shown by this contemporary Russian short form table, which includes all elements and element names until roentgenium.
H. G. Deming used the so-called long periodic table (18 columns) in his textbook "General Chemistry", which appeared in the US for the first time in 1923 (Wiley), and was the first to designate the first two and the last five main groups with the notation "A", and the intervening transition groups with the notation "B".
The numeration was chosen so that the characteristic oxides of the B groups would correspond to those of the A groups. The iron, cobalt, and nickel groups were designated neither A nor B. The noble-gas group was originally attached (by Deming) to the left side of the periodic table. The group was later switched to the right side and usually labeled as group VIIIA.
Extension of the periodic table
In the extended periodic table, suggested by Glenn T. Seaborg in 1969, yet unknown elements are included up to atomic number 168. Theoretical periods above regular period 7 are added.
In the research field of superatoms, clusters of atoms have properties of single atoms of another element. It is suggested to extend the periodic table with a second layer to be occupied with these cluster compounds. The latest addition to this multi-story table is the aluminium cluster ion Al−
7, which behaves like a multivalent germanium atom.[15]
Gallery
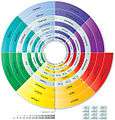 Spiral periodic table (Robert W Harrison)
Spiral periodic table (Robert W Harrison).png) The Ring Of Periodic Elements (TROPE)
The Ring Of Periodic Elements (TROPE)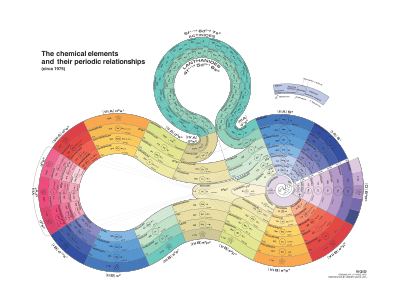 Curled ribbon periodic table (J. F. Hyde)
Curled ribbon periodic table (J. F. Hyde) Circular periodic table
Circular periodic table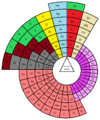 Alternative circular periodic table
Alternative circular periodic table.SVG.png) Spiral periodic table (Jan Scholten)
Spiral periodic table (Jan Scholten) Mendeleev's Flower (Flower periodic table)
Mendeleev's Flower (Flower periodic table) Binary electron shells periodic table
Binary electron shells periodic table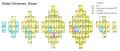 "Stowe" periodic table
"Stowe" periodic table "Zmaczynski & Bayley" periodic table
"Zmaczynski & Bayley" periodic table.svg.png) ADOMAH periodic table (V. Tsimmerman)
ADOMAH periodic table (V. Tsimmerman).jpg) Newlands revisited
Newlands revisited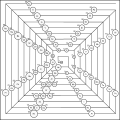 Pyramidal periodic table
Pyramidal periodic table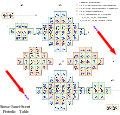 Stowe–Janet–Scerri with 3D electron orbitals
Stowe–Janet–Scerri with 3D electron orbitals 4D Stowe–Janet–Scerri periodic table
4D Stowe–Janet–Scerri periodic table
References
- E. R. Scerri. The Periodic Table, Its Story and Its Significance. Oxford University Press, New York, 2006, ISBN 0195345673.
- Henry Bent. New Ideas in Chemistry from Fresh Energy for the Periodic Law. AuthorHouse, 2006, ISBN 978-1-4259-4862-7.
- "Left Step Periodic Table". 1928. Retrieved 2014-02-15.
- Stewart, Philip J. (2009). "Charles Janet: Unrecognized genius of the periodic system". Foundations of Chemistry. 12: 5–15. doi:10.1007/s10698-008-9062-5.
- Benfey's table appears in an article by Glenn Seaborg, "Plutonium: The Ornery Element", Chemistry, June 1964, 37 (6), 12–17, on p. 14.
- Mazurs, E. G. (1974). Graphical Representations of the Periodic System During One Hundred Years. Alabama: University of Alabama Press. p. 111. ISBN 978-0-8173-3200-6.
- The animated depiction of Giguère's periodic table that is widely available on the internet (including here) is erroneous, as it does not include hydrogen and helium. Giguère included hydrogen, above lithium, and helium, above beryllium. See Giguère P. A. (1966). "The 'new look' for the periodic system". Chemistry in Canada vol. 18 (12): 36–39 (see p. 37).
- Stowe, Timothy. "Physicist's Periodic Table 1989". jeries.rihani.com. Retrieved 24 September 2019.
A physicist's chart of the chemical elements
- Bradley, David (20 July 2011). "At Last, A Definitive Periodic Table?". Chemviews. ChemistryViews.org. doi:10.1002/chemv.201000107. Retrieved 24 September 2019.
- Rich, Ronald L. (2005). "Are Some Elements More Equal Than Others?". J. Chem. Educ. 82 (12): 1761. Bibcode:2005JChEd..82.1761R. doi:10.1021/ed082p1761.
- Clancy, Christina; et al. (2010). Chemistry 11. Canada: McGraw-Hill Ryerson. p. 28. ISBN 978-007091575-6.
- Leach, M. R. "ADOMAH Periodic Table by Valery Tsimmerman". Internet Database of Periodic Tables. Retrieved July 29, 2019.; Stewart, P. J. (2010). "Charles Janet: unrecognized genius of the periodic system". Foundations of Chemistry. 12 (1): 5–15. doi:10.1007/s10698-008-9062-5.
- Stewart, P. (2018). "Amateurs and professionals in chemistry: The case of the periodic table". In Scerri, E.; Restrepo, G. (eds.). From Mendeleev to Oganesson: A Multidisciplinary Perspective on the Periodic Table. New York: Oxford University Press. pp. 66–79 (76–77). ISBN 978-0-190-66853-2.; Leach, M. R. "ADOMAH Periodic Table Glass Cube". Internet Database of Periodic Tables. Retrieved August 1, 2019.
- Marks, E. G.; Marks, J. A. (2010). "Newlands revisited: A display of the periodicity of the chemical elements for chemists". Foundations of Chemistry. 12: 85–93. doi:10.1007/s10698-010-9083-8.
- Amato, Ivan (November 21, 2006). "Beyond The Periodic Table Metal clusters mimic chemical properties of atoms". Chemical & Engineering News.
Further reading
- A 1974 review of the tables then known is considered a definitive work on the topic:[1] Mazurs, E. G. Graphical Representations of the Periodic System During One Hundred Years. Alabama; University of Alabama Press, 1974, ISBN 0-8173-3200-6.
- Hjørland, Birger (2011). The periodic table and the philosophy of classification. Knowledge Organization, 38(1), 9–21.
External links
- Representing the Periodic Table in Different Ways a site curated by the Michigan State University Alumni Association Knowledge Network
- Robert Harrison's modern spiral periodic table
- Janet's Left Step Periodic Table
- Correction to Physicist periodic table offered by Jeries Rihani as Meitnerium occupies the position that Hassium should have.
- A Wired Article on Alternate Periodic Tables
- A Selection of Periodic Tables
- http://periodicspiral.com/ arranges the periodic table in a (hexagonal) spiral.
- Rotaperiod.com A new periodic table.
- Note on the T-shirt topology of the Z-spiral.
- New Periodic Table of the Elements this is in a square-triangular periodic arrangement.
- Periodic Table based on electron configurations
- Database of Periodic Tables
- Periodic Fractal of the Elements
- Bob Doyle Periodic Table of the Elements A regrouping by properties used to better explain electron grouping
- Earth Scientist's Periodic Table
.svg.png)