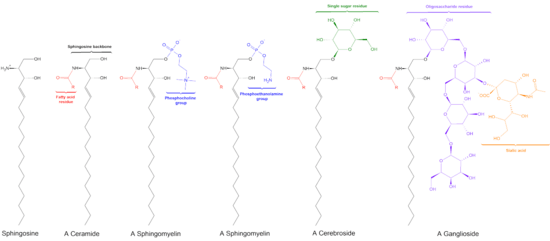
Ceramide
Ceramides are a family of waxy lipid molecules. A ceramide is composed of sphingosine and a fatty acid. Ceramides are found in high concentrations within the cell membrane of eukaryotic cells, since they are component lipids that make up sphingomyelin, one of the major lipids in the lipid bilayer.[1] Contrary to previous assumptions that ceramides and other sphingolipids found in cell membrane were purely supporting structural elements, ceramide can participate in a variety of cellular signaling: examples include regulating differentiation, proliferation, and programmed cell death (PCD) of cells.
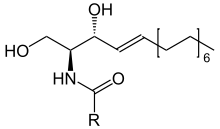
The word ceramide comes from the Latin cera (wax) and amide. Ceramide is a component of vernix caseosa, the waxy or cheese-like white substance found coating the skin of newborn human infants.
Pathways for ceramide synthesis
There are three major pathways of ceramide generation. First, the sphingomyelinase pathway uses an enzyme to break down sphingomyelin in the cell membrane and release ceramide. Second, the de novo pathway creates ceramide from less complex molecules. Third, in the "salvage" pathway, sphingolipids that are broken down into sphingosine are reused by reacylation to form ceramide.
Sphingomyelin hydrolysis
Hydrolysis of sphingomyelin is catalyzed by the enzyme sphingomyelinase. Because sphingomyelin is one of the four common phospholipids found in the plasma membrane of cells, the implications of this method of generating ceramide is that the cellular membrane is the target of extracellular signals leading to programmed cell death. There has been research suggesting that when ionizing radiation causes apoptosis in some cells, the radiation leads to the activation of sphingomyelinase in the cell membrane and ultimately, to ceramide generation.[2]
De novo
De novo synthesis of ceramide begins with the condensation of palmitate and serine to form 3-keto-dihydrosphingosine. This reaction is catalyzed by the enzyme serine palmitoyl transferase and is the rate-limiting step of the pathway. In turn, 3-keto-dihydrosphingosine is reduced to dihydrosphingosine, which is then followed by acylation by the enzyme (dihydro)ceramide synthase to produce dihydroceramide. The final reaction to produce ceramide is catalyzed by dihydroceramide desaturase. De novo synthesis of ceramide occurs in the endoplasmic reticulum. Ceramide is subsequently transported to the Golgi apparatus by either vesicular trafficking or the ceramide transfer protein CERT. Once in the Golgi apparatus, ceramide can be further metabolized to other sphingolipids, such as sphingomyelin and the complex glycosphingolipids.[3]
Salvage pathway
Constitutive degradation of sphingolipids and glycosphingolipids takes place in the acidic subcellular compartments, the late endosomes and the lysosomes, with the end goal of producing sphingosine. In the case of glycosphingolipids, exohydrolases acting at acidic pH optima cause the stepwise release of monosaccharide units from the end of the oligosaccharide chains, leaving just the sphingosine portion of the molecule, which may then contribute to the generation of ceramides. Ceramide can be further hydrolyzed by acid ceramidase to form sphingosine and a free fatty acid, both of which are able to leave the lysosome, unlike ceramide. The long-chain sphingoid bases released from the lysosome may then re-enter pathways for synthesis of ceramide and/or sphingosine-1-phosphate. The salvage pathway re-utilizes long-chain sphingoid bases to form ceramide through the action of ceramide synthase. Thus, ceramide synthase family members probably trap free sphingosine released from the lysosome at the surface of the endoplasmic reticulum or in endoplasmic reticulum-associated membranes. The salvage pathway has been estimated to contribute from 50% to 90% of sphingolipid biosynthesis.[4]
Physiological roles
As a bioactive lipid, ceramide has been implicated in a variety of physiological functions including apoptosis, cell growth arrest, differentiation, cell senescence, cell migration and adhesion.[3] Roles for ceramide and its downstream metabolites have also been suggested in a number of pathological states including cancer, neurodegeneration, diabetes, microbial pathogenesis, obesity, and inflammation.[5][6]
Ceramides induce skeletal muscle insulin resistance when synthesized as a result of saturated fat activation of TLR4 receptors.[7] Unsaturated fat does not have this effect.[7] Ceramides induce insulin resistance in many tissues by inhibition of Akt/PKB signaling.[8] Aggregation of LDL cholesterol by ceramide causes LDL retention in arterial walls, leading to atherosclerosis.[9] Ceramides cause endothelial dysfunction by activating protein phosphatase 2 (PP2A).[10] In mitochondria, ceramide suppresses the electron transport chain and induces production of reactive oxygen species.[11]
Apoptosis
One of the most studied roles of ceramide pertains to its function as a proapoptotic molecule. Apoptosis, or Type I programmed cell death, is essential for the maintenance of normal cellular homeostasis and is an important physiological response to many forms of cellular stress. Ceramide accumulation has been found following treatment of cells with a number of apoptotic agents including ionizing radiation,[2][12] UV light,[13] TNF-alpha,[14] and chemotherapeutic agents. This suggests a role for ceramide in the biological responses of all these agents. Because of its apoptosis-inducing effects in cancer cells, ceramide has been termed the "tumor suppressor lipid". Several studies have attempted to define further the specific role of ceramide in the events of cell death and some evidence suggests ceramide functions upstream of the mitochondria in inducing apoptosis. However, owing to the conflicting and variable nature of studies into the role of ceramide in apoptosis, the mechanism by which this lipid regulates apoptosis remains elusive.[15]
Skin
Ceramide is the main component of the stratum corneum of the epidermis layer of human skin.[16][17] Together with cholesterol and saturated fatty acids, ceramide creates a water-impermeable, protective organ to prevent excessive water loss due to evaporation as well as a barrier against the entry of microorganisms.[17] In the hyperplastic disorder psoriasis the water permeability barrier is compromised.[18] Ceramide VI is the most abundant ceramide of the skin, along with ceramide II, and has been exploited to model the organization of the stratum corneum lipidic network.[19][20]
The stratum corneum is composed of 50% ceramides, 25% cholesterol, and 15% free fatty acids.[18] Key components of the extracellular lipid lamellae of the stratum corneum are ultra long chain (C28-C36) ceramides.[21] With aging there is a decline in ceramide and cholesterol in the stratum corneum of humans.[22] A clinical trial using ceramide-rich wheat extract showed increased skin hydration in those taking the extract rather than the placebo.[23]
Hormonal
Inhibition of ceramide synthesis with myriocin in obese mice may lead to both improved leptin signaling and decreased insulin resistance by decreasing SOCS-3 expression.[24] An elevated level of ceramide can cause insulin resistance by inhibiting the ability of insulin to activate the insulin signal transduction pathway and/or via the activation of JNK.[25]
Substances known to induce ceramide generation
- Anandamide
- Ceramidase inhibitors
- Chemotherapeutic agents
- Fas ligand
- Endotoxin
- Homocysteine[26]
- Heat
- Gamma interferon
- Ionizing radiation[2][27]
- Matrix metalloproteinases[26]
- Niacinamide
- Reactive oxygen species[26]
- Tetrahydrocannabinol and other cannabinoids[28]
- TNF-alpha[26]
- 1,25 Dihydroxy vitamin D
Mechanism by which ceramide signaling occurs
Currently, the means by which ceramide acts as a signaling molecule are not clear.
One hypothesis is that ceramide generated in the plasma membrane enhances membrane rigidity and stabilizes smaller lipid platforms known as lipid rafts, allowing them to serve as platforms for signalling molecules. Moreover, as rafts on one leaflet of the membrane can induce localized changes in the other leaflet of the bilayer, they can potentially serve as the link between signals from outside the cell to signals to be generated within the cell.
Ceramide has also been shown to form organized large channels traversing the mitochondrial outer membrane. This leads to the egress of proteins from the intermembrane space.[29][30][31]
Uses
Ceramides may be found as ingredients of some topical skin medications used to complement treatment for skin conditions such as eczema.[32] They are also used in cosmetic products such as some soaps, shampoos, skin creams, and sunscreens.[33] Additionally, ceramides are being explored as a potential therapeutic in cancer.[34]
Ceramide in bacteria
Ceramide is rarely found in bacteria.[35] Bacteria of family Sphingomonadaceae, however, contain it.
References
- Davis, Deanna; Kannan, Muthukumar; Wattenberg, Binks (2018-12-01). "Orm/ORMDL proteins: Gate guardians and master regulators". Advances in Biological Regulation. Sphingolipid Signaling in Chronic Disease. 70: 3–18. doi:10.1016/j.jbior.2018.08.002. ISSN 2212-4926. PMC 6251742. PMID 30193828.
- Haimovitz-Friedman A, Kan CC, Ehleiter D, et al. (1994). "Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis". J. Exp. Med. 180 (2): 525–35. doi:10.1084/jem.180.2.525. PMC 2191598. PMID 8046331.
- Hannun, Y.A.; Obeid, L.M. (2008). "Principles of bioactive lipid signalling: lessons from sphingolipids". Nature Reviews Molecular Cell Biology. 9 (2): 139–150. doi:10.1038/nrm2329. PMID 18216770.
- Kitatani K, Idkowiak-Baldys J, Hannun YA (2008). "The sphingolipid salvage pathway in ceramide metabolism and signaling". Cell Signaling. 20 (6): 1010–1018. doi:10.1016/j.cellsig.2007.12.006. PMC 2422835. PMID 18191382.
- Zeidan, Y.H.; Hannun, Y.A. (2007). "Translational aspects of sphingolipid metabolism". Trends Mol. Med. 13 (8): 327–336. doi:10.1016/j.molmed.2007.06.002. PMID 17588815.
- Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN (2007). "Aging up-regulates expression of inflammatory mediators in mouse adipose tissue". The Journal of Immunology. 179 (7): 4829–39. doi:10.4049/jimmunol.179.7.4829. PMID 17878382.
- Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA (2011). "Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice". Journal of Clinical Investigation. 121 (5): 1858–1870. doi:10.1172/JCI43378. PMC 3083776. PMID 21490391.
- Chavez JA, Siddique MM, Wang ST, Ching J, Shayman JA, Summers SA (2014). "Ceramides and glucosylceramides are independent antagonists of insulin signaling". Cell. 289 (2): 723–734. doi:10.1074/jbc.M113.522847. PMC 3887200. PMID 24214972.
- Li Z, Basterr MJ, Hailemariam TK, Hojjati MR, Lu S, Liu J, Liu R, Zhou H, Jiang XC (2005). "The effect of dietary sphingolipids on plasma sphingomyelin metabolism and atherosclerosis". Biochimica et Biophysica Acta. 1735 (2): 130–134. doi:10.1016/j.bbalip.2005.05.004. PMID 15967715.
- Mehra VC, Jackson E, Zhang XM, Jiang XC, Dobrucki LW, Yu J, Bernatchez P, Sinusas AJ, Shulman GI, Sessa WC, Yarovinsky TO, Bender JR (2014). "Ceramide-activated phosphatase mediates fatty acid-induced endothelial VEGF resistance and impaired angiogenesis". The American Journal of Pathology. 184 (5): 1562–1576. doi:10.1016/j.ajpath.2014.01.009. PMC 4005977. PMID 24606881.
- Kogot-Levin A, Saada A (2014). "Ceramide and the mitochondrial respiratory chain". Biochimie. 100: 88–94. doi:10.1016/j.biochi.2013.07.027. PMID 23933096.
- Dbaibo GS, Pushkareva MY, Rachid RA, Alter N, Smyth MJ, Obeid LM, Hannun YA (1998). "p53-dependent ceramide response to genotoxic stress". J. Clin. Invest. 102 (2): 329–339. doi:10.1172/JCI1180. PMC 508891. PMID 9664074.
- Rotolo JA, Zhang J, Donepudi M, Lee H, Fuks Z, Kolesnick R (2005). "Caspase-dependent and -independent activation of acid sphingomyelinase signaling". J. Biol. Chem. 280 (28): 26425–34. doi:10.1074/jbc.M414569200. PMID 15849201.
- Dbaibo GS, El-Assaad W, Krikorian A, Liu B, Diab K, Idriss NZ, El-Sabban M, Driscoll TA, Perry DK, Hannun YA (2001). "Ceramide generation by two distinct pathways in tumor necrosis factor alpha-induced cell death". FEBS Letters. 503 (1): 7–12. doi:10.1016/S0014-5793(01)02625-4. PMID 11513845.
- Taha TA, Mullen TD, Obeid LM (2006). "A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death". Biochim. Biophys. Acta. 1758 (12): 2027–36. doi:10.1016/j.bbamem.2006.10.018. PMC 1766198. PMID 17161984.
- Hill JR, Wertz PW (2009). "Structures of the ceramides from porcine palatal stratum corneum". Lipids. 44 (3): 291–295. doi:10.1007/s11745-009-3283-9. PMID 19184160.
- Garidel P, Fölting B, Schaller I, Kerth A (2010). "The microstructure of the stratum corneum lipid barrier: mid-infrared spectroscopic studies of hydrated ceramide:palmitic acid:cholesterol model systems". Biophysical Chemistry. 150 (1–3): 144–156. doi:10.1016/j.bpc.2010.03.008. PMID 20457485.
- Feingold KR (2007). "Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis". Journal of Lipid Research. 48 (12): 2531–2546. doi:10.1194/jlr.R700013-JLR200. PMID 17872588.
- Musazzi, Umberto M.; Matera, Carlo; Dallanoce, Clelia; Vacondio, Federica; De Amici, Marco; Vistoli, Giulio; Cilurzo, Francesco; Minghetti, Paola (2015). "On the selection of an opioid for local skin analgesia: Structure-skin permeability relationships". International Journal of Pharmaceutics. 489 (1–2): 177–185. doi:10.1016/j.ijpharm.2015.04.071. ISSN 0378-5173. PMID 25934430.
- Wertz, Philip W.; van den Bergh, Benedicte (1998). "The physical, chemical and functional properties of lipids in the skin and other biological barriers". Chemistry and Physics of Lipids. 91 (2): 85–96. doi:10.1016/S0009-3084(97)00108-4. ISSN 0009-3084. PMID 9569614.
- Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, Rothermel U, Bayerle A, van der Hoeven F, Imgrund S, Kirsch J, Nickel W, Willecke K, Riezman H, Gröne HJ, Sandhoff R (2012). "Loss of ceramide synthase 3 causes lethal skin barrier disruption" (PDF). Human Molecular Genetics. 21 (3): 586–608. doi:10.1093/hmg/ddr494. PMID 22038835.
- Popa I, Abdul-Malak N, Portoukalian J (2010). "The weak rate of sphingolipid biosynthesis shown by basal keratinocytes isolated from aged vs. young donors is fully rejuvenated after treatment with peptides of a potato hydrolysate". International Journal of Cosmetic Science. 32 (3): 225–232. doi:10.1111/j.1468-2494.2009.00571.x. PMID 20384897.
- Guillou S, Ghabri S, Jannot C, Gaillard E, Lamour I, Boisnic S (2011). "The moisturizing effect of a wheat extract food supplement on women's skin: a randomized, double-blind placebo-controlled trial" (PDF). International Journal of Cosmetic Science. 33 (2): 138–143. doi:10.1111/j.1468-2494.2010.00600.x. PMID 20646083. Archived from the original (PDF) on 2016-01-16. Retrieved 2017-06-07.
- Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F (2009). "Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome". American Journal of Physiology. 297 (1): E211–E224. doi:10.1152/ajpendo.91014.2008. PMC 2711669. PMID 19435851.
- Febbraio, Mark (2014). "Role of interleukins in obesity:implications for metabolic disease". Trends in Endocrinology and Metabolism. 25 (6): 312–319. doi:10.1016/j.tem.2014.02.004. PMID 24698032.
- Bismuth J, Lin P, Yao Q, Chen C (2008). "Ceramide: a common pathway for atherosclerosis?". Atherosclerosis. 196 (2): 497–504. doi:10.1016/j.atherosclerosis.2007.09.018. PMC 2924671. PMID 17963772.
- Hallahan DE (1996). "Radiation-mediated gene expression in the pathogenesis of the clinical radiation response". Sem. Radiat. Oncol. 6 (4): 250–267. doi:10.1016/S1053-4296(96)80021-X. PMID 10717183.
- Velasco, G; Galve-Roperh, I; Sánchez, C; Blázquez, C; Haro, A; Guzmán, M (2005). "Cannabinoids and ceramide: Two lipids acting hand-by-hand". Life Sciences. 77 (14): 1723–31. doi:10.1016/j.lfs.2005.05.015. PMID 15958274.
- Siskind LJ, Kolesnick RN, Colombini M (2002). "Ceramide Channels Increase the Permeability of the Mitochondrial Outer Membrane to Small Proteins". J. Biol. Chem. 277 (30): 26796–803. doi:10.1074/jbc.M200754200. PMC 2246046. PMID 12006562.
- Stiban J, Fistere D, Colombini M (2006). "Dihydroceramide hinders ceramide channel formation: Implications on apoptosis". Apoptosis. 11 (5): 773–80. doi:10.1007/s10495-006-5882-8. PMID 16532372.
- Siskind LJ, Kolesnick RN, Colombini M (2006). "Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations". Mitochondrion. 6 (3): 118–25. doi:10.1016/j.mito.2006.03.002. PMC 2246045. PMID 16713754.
- "Ceramides - Skin Lipids That Keep Skin Moisturized". Retrieved 29 January 2015.
- "Safety Assessment of Ceramides as Used in Cosmetics" (PDF). Cosmetic Ingredient Review. May 16, 2014. Cite journal requires
|journal=(help) - Huang, WC; Chen, CL; Lin, YS; Lin, CF (2011). "Apoptotic Sphingolipid Ceramide in Cancer Therapy". Journal of Lipids. 2011 (2011): 565316. doi:10.1155/2011/565316. PMC 3066853. PMID 21490804.
- Minamino, Miki; Sakaguchi, Ikuyo; Naka, Takashi; Ikeda, Norikazu; Kato, Yoshiko; Tomiyasu, Ikuko; Yano, Ikuya; Kobayashi, Kazuo (2003). "Bacterial ceramides and sphingophospholipids induce apoptosis of human leukaemic cells". Microbiology. 149 (8): 2071–2081. doi:10.1099/mic.0.25922-0. PMID 12904547.
External links
- Ceramides at the US National Library of Medicine Medical Subject Headings (MeSH)