GD2
GD2 is a disialoganglioside expressed on tumors of neuroectodermal origin, including human neuroblastoma and melanoma, with highly restricted expression on normal tissues, principally to the cerebellum and peripheral nerves in humans.
 | |
| Names | |
|---|---|
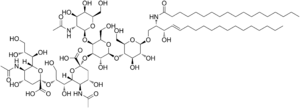
| IUPAC name
(2R,4R,5S,6S)-2-[3-[(2S,3S,4R,6S)-6-[(2S,3R,4R,5S,6R)-5-[(2S,3R,4R,5R,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-2-[(2R,3S,4R,5R,6R)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(E)-3-hydroxy-2-(octadecanoylamino)octadec-4-enoxy]oxan-3-yl]oxy-3-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3-amino-6-carboxy-4-hydroxyoxan-2-yl]-2,3-dihydroxypropoxy]-5-amino-4-hydroxy-6-(1,2,3-trihydroxypropyl)oxane-2-carboxylic acid | |
| Other names
Ganglioside G2 | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C74H134N4O32 | |
| Molar mass | 1591.882 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The relatively tumor-specific expression of GD2 makes it a suitable target for immunotherapy with monoclonal antibodies or with artificial T cell receptors.[1] An example of such antibodies is hu14.18K322A, a monoclonal antibody. This anti-GD2 antibody is currently undergoing a phase II clinical trial in the treatment of previously untreated high risk neuroblastoma given alongside combination chemotherapy prior to stem cell transplant and radiation therapy.[2] A prior phase I clinical trial for patients with refractory or recurrent neuroblastoma designed to decrease toxicity found safe dosage amounts and determined that common toxicities, particularly pain, could be well managed. [3]
See also
References
- Wierzbicki, Andrzej; Gil, Margaret; Ciesielski, Michael; Fenstermaker, Robert A.; Kaneko, Yutaro; Rokita, Hanna; Lau, Joseph T.; Kozbor, Danuta (2008). "Immunization with a Mimotope of GD2 Ganglioside Induces CD8+ T Cells That Recognize Cell Adhesion Molecules on Tumor Cells". Journal of Immunology. 181 (9): 6644–6653. doi:10.4049/jimmunol.181.9.6644. PMC 2730120. PMID 18941255.
- "Clinical trials using anti-GD2 monoclonal antibody hu14.18K322A". National Cancer Institute. Retrieved April 20, 2018.
- Navid F, et al. (May 2014). "Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma". Journal of Clinical Oncology. 32 (14): 1445–52. doi:10.1200/JCO.2013.50.4423. PMC 4017710. PMID 24711551.
2. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. Ahmed M, Cheung NK. FEBS Lett. 2014 Jan 21;588(2):288-97
3. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Cheung NK, Dyer MA. Nat Rev Cancer. 2013 Jun;13(6):397-411