Magnesium silicide
Magnesium silicide, Mg2Si, is an inorganic compound consisting of magnesium and silicon. As-grown Mg2Si usually forms black crystals; they are semiconductors with n-type conductivity and have potential applications in thermoelectric generators.[3]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Magnesium silicide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.041.125 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Mg2Si | |
| Molar mass | 76.695 g·mol−1 |
| Appearance | Gray cubic crystals[1] |
| Density | 1.99 g cm−3[1] |
| Melting point | 1,102 °C (2,016 °F; 1,375 K)[1] |
| reacts[1] | |
| Structure[2] | |
| Cubic, cF12 | |
| Fm3m, #225 | |
a = 0.6351 nm | |
Formula units (Z) |
4 |
| Hazards | |
| Main hazards | reacts with hydrochloric acid to produce silane |
| R-phrases (outdated) | R23, R24, R25, R34 |
| Related compounds | |
Other cations |
Calcium silicide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
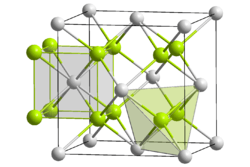
Crystal structure
Mg2Si crystallizes in the antifluorite structure. In the face-centered cubic lattice Si centers occupy the corners and face-centered positions of the unit cell and Mg centers occupy eight tetrahedral sites in the interior of the unit cell. The coordination numbers of Si and Mg are eight and four, respectively.[2]
Synthesis
It can be produced by heating silicon dioxide, SiO2, found in sand, with excess magnesium. The process first forms silicon metal and magnesium oxide, and, if an excess of SiO2 is used, then elemental silicon is formed:
- 2 Mg + SiO2 → 2 MgO + Si
If an excess of Mg is present, Mg2Si is formed from the reaction of the remaining magnesium with the silicon:
- 2 Mg + Si → Mg2Si
These reactions proceed exothermically.[4]
Reactions
Magnesium silicide can be viewed as consisting of Si4− ions. As such it is reactive toward acids. Thus, when magnesium silicide is treated with hydrochloric acid, silane (SiH4) and magnesium chloride are produced:
- Mg2Si + 4 HCl → SiH4 + 2 MgCl2
Sulfuric acid can be used as well. These protonolysis reactions are typical of a Group 2 and Group 1 silicides.
Uses
Magnesium silicide is used to create aluminium alloys of the 6000 series, containing up to approximately 1.5% Mg2Si. An alloy of this group can be age-hardened to form Guinier-Preston zones and a very fine precipitate, both resulting in increased strength of the alloy.[5]
Magnesium silicide is a narrow-gap semiconductor. Its as-grown crystal exhibit n-type conductivity, but it can changed to p-type by doping with Ag, Ga, Sn and possibly Li (at high doping level). The major potential electronic application of Mg2Si is in thermoelectric generators.[3][6]
References
| Wikimedia Commons has media related to Magnesium silicide. |
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.74. ISBN 1439855110.
- Noda Y., Kon H., Furukawa Y., Otsuka N., Nishida I.A., Masumoto K. (1992). "Preparation and Thermoelectric Properties of Mg2Si1−xGex (x=0.0∼0.4) Solid Solution Semiconductors". Mater. Trans., JIM. 33 (9): 845–850. doi:10.2320/matertrans1989.33.845.CS1 maint: multiple names: authors list (link)
- Hirayama, Naomi (2019). "Substitutional and interstitial impurity p-type doping of thermoelectric Mg2Si: a theoretical study". Sci. Technol. Adv. Mater. 20 (1): 160–172. doi:10.1080/14686996.2019.1580537. PMC 6419642. PMID 30891103.

- Ehrlich, P. (1963) "Alkaline Earth Metals", p. 920 in Handbook of Preparative Inorganic Chemistry, 2nd ed., Vol. 1. G. Brauer (ed.). Academic Press, New York.
- "Properties and Selection: Non-ferrous Alloys and Special Purpose Materials" in ASM Handbook, 10th ed., Vol. 1, 1990, ASM International, Materials Park, Ohio. ISBN 0871703785.
- Borisenko, Victor E. (2013). Semiconducting Silicides: Basics, Formation, Properties. Springer Science & Business Media. pp. 187, 287. ISBN 978-3-642-59649-0.