Magnesium polonide
Magnesium polonide (MgPo) is a salt of magnesium and polonium. It is a polonide, a set of very chemically stable compounds of polonium.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Magnesium polonide | |
| Identifiers | |
| Properties | |
| MgPo | |
| Molar mass | 233.29 g/mol |
| Appearance | greyish[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Magnesium polonide may be produced by heating elemental magnesium and polonium together at 300–400 °C.[1]
Structure
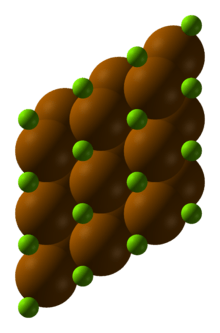
Magnesium polonide has the nickeline (NiAs) structure with lattice parameters a = 434.5 pm and c = 707.7 pm.[1][2] It is unusual among polonides in not being isomorphous with the corresponding sulfide, selenide and telluride; only mercury polonide (HgPo) shares this property.[3]
gollark: "Kura Technologies" apparently managed some very impressive things with accursed physicsy optics, but I don't think they're widely available now or have had proper external reviews.
gollark: I'm mostly interested in AR, since a better interface for looking at internet things on the go than my phone would be very convenient.
gollark: Yet most people in developed countries apparently have smartphones now.
gollark: Presumably if they get particularly popular, they'll be available on the technically-worse-but-better-looking-than-buying-it-outright phone-style contracts.
gollark: ↑
References
- Bagnall, K. W. (1962). "The Chemistry of Polonium". Advances in Inorganic Chemistry and Radiochemistry. New York: Academic Press. pp. 197–230. ISBN 9780120236046. Retrieved June 17, 2012.
- Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 899. ISBN 978-0-08-022057-4.
- Witteman, W. G.; Giorgi, A. L.; Vier, D. T. (1960). "The Preparation and Identification of Some Intermetallic Compounds of Polonium". Journal of Physical Chemistry. American Chemical Society. 64 (4): 434–440. doi:10.1021/j100833a014. OSTI 4190680.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.