Laquinimod
Laquinimod is an experimental immunomodulator developed by Active Biotech and Teva. It is being investigated as an oral treatment for multiple sclerosis (MS).
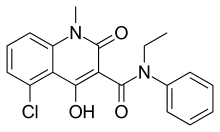 | |
| Names | |
|---|---|
| IUPAC name
5-chloro-N-ethyl-4-hydroxy-1-methyl-2-oxo- | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.219.958 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H17ClN2O3 | |
| Molar mass | 356.803 g/mol |
| Pharmacology | |
| N07XX10 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Laquinimod is the successor of Active Biotech's failed experimental immunomodulator linomide.[1]
The compound has been investigated in two Phase II trials using successive magnetic resonance scans (MRI). Laquinimod seems to be able to reduce the MS disease activity on MRI.[2][3][4] However, the response to a given dose was discrepant between both studies.[5]
Phase III studies for MS started in December 2007.[6] In 2011, Teva announced its clinical trials involving laquinimod had failed, being unable to significantly reduce relapses in MS among patients beyond a placebo.[7] However, the final results of above-mentioned phase III trial proved oral laquinimod administered once daily slowed the progression of disability and reduced the rate of relapse in patients with relapsing–remitting multiple sclerosis.[8]
On May 7, 2013 laquinimod was approved by the Russian Ministry of Health (the FDA analog) as a treatment for relapsing-remitting multiple sclerosis (RRMS) under the brand name Nerventra.[9]
See also
- Management of multiple sclerosis
- Fingolimod, a marketed drug for MS with a different mechanism
- Ponesimod, an experimental drug for MS with a different mechanism
References
- Tan IL, Lycklama à Nijeholt GJ, Polman CH, et al. (April 2000). "Linomide in the treatment of multiple sclerosis: MRI results from prematurely terminated phase-III trials". Mult Scler. 6 (2): 99–104. doi:10.1191/135245800678827626. PMID 10773855.
- Comi G, Pulizzi A, Rovaris M, et al. (June 2008). "Effect of laquinimod on MRI-monitored disease activity in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study". Lancet. 371 (9630): 2085–2092. doi:10.1016/S0140-6736(08)60918-6. PMID 18572078.
- Polman C, Barkhof F, Sandberg-Wollheim M, et al. (March 2005). "Treatment with laquinimod reduces development of active MRI lesions in relapsing MS". Neurology. 64 (6): 987–91. doi:10.1212/01.WNL.0000154520.48391.69. PMID 15781813.
- He, Dian; Han, Kai; Gao, Xiangdong; Dong, Shuai; Chu, Lan; Feng, ZhanHui; Wu, Shan (2013-08-06). Chu, Lan (ed.). "Laquinimod for multiple sclerosis". The Cochrane Database of Systematic Reviews (8): CD010475. doi:10.1002/14651858.CD010475.pub2. ISSN 1469-493X. PMID 23922214.
- Keegan BM, Weinshenker BG (June 2008). "Laquinimod, a new oral drug for multiple sclerosis". Lancet. 371 (9630): 2059–2060. doi:10.1016/S0140-6736(08)60894-6. PMID 18572062.
- Clinical trial number NCT00509145 for "Safety and Efficacy of Orally Administered Laquinimod Versus Placebo for Treatment of Relapsing Remitting Multiple Sclerosis (RRMS) (ALLEGRO)" at ClinicalTrials.gov
- Kresege, Naomi (1 August 2011). "Teva's Copaxone Successor Fails in Latest Clinical Trial". Bloomberg. Retrieved 2 August 2011.
Teva Pharmaceutical Industries Ltd. (TEVA)’s experimental multiple sclerosis pill failed to reduce relapses more than placebo in a clinical trial, dealing a blow to the company’s effort to find a successor to an older drug.
- Comi, G.; Jeffery, D.; Kappos, L.; Montalban, X.; Boyko, A.; Rocca, M. A.; Filippi, M.; Allegro Study, G. (2012). "Placebo-Controlled Trial of Oral Laquinimod for Multiple Sclerosis". New England Journal of Medicine. 366 (11): 1000–1009. doi:10.1056/NEJMoa1104318. hdl:10459.1/65687. PMID 22417253.
- "Nerventra (laquinimod) Capsules 0,6 mg. Registration certificate". State Register of Medicines (in Russian). Retrieved 21 October 2015.