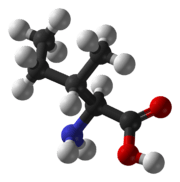
Isoleucine
Isoleucine (symbol Ile or I)[1] is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes in other organisms such as bacteria.[2] It is encoded by the codons AUU, AUC, and AUA.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Isoleucine | |||
| Other names
(2S,3S)-2-amino-3-methylpentanoic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.726 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H13NO2 | |||
| Molar mass | 131.175 g·mol−1 | ||
| −84.9·10−6 cm3/mol | |||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Inability to break down isoleucine, along with other amino acids, is associated with maple syrup urine disease.[3]
Metabolism
Biosynthesis
As an essential nutrient, it is not synthesized in the body, hence it must be ingested, usually as a component of proteins. In plants and microorganisms, it is synthesized via several steps, starting from pyruvate and alpha-ketobutyrate. Enzymes involved in this biosynthesis include:[4]
- Acetolactate synthase (also known as acetohydroxy acid synthase)
- Acetohydroxy acid isomeroreductase
- Dihydroxyacid dehydratase
- Valine aminotransferase
Catabolism
Isoleucine is both a glucogenic and a ketogenic amino acid. After transamination with alpha-ketoglutarate the carbon skeleton is oxidised and split into propionyl-CoA and acetyl-CoA. Propionyl-CoA is converted into succinyl-CoA, a TCA cycle intermediate which can be converted into oxaloacetate for gluconeogenesis (hence glucogenic). In mammals acetyl-CoA cannot be converted to carbohydrate but can be either fed into the TCA cycle by condensing with oxaloacetate to form citrate or used in the synthesis of ketone bodies (hence ketogenic) or fatty acids.[5]
Insulin resistance
Isoleucine, like other branched-chain amino acids, is associated with insulin resistance: higher levels of isoleucine are observed in the blood of diabetic mice, rats, and humans.[6] Mice fed an isoleucine deprivation diet for one day have improved insulin sensitivity, and feeding of an isoleucine deprivation diet for one week significantly decreases blood glucose levels.[7] In diet-induced obese and insulin resistant mice, a diet with decreased levels of isoleucine and the other branched-chain amino acids results in reduced adiposity and improved insulin sensitivity.[8] In humans, a protein restricted diet lowers blood levels of isoleucine and decreases fasting blood glucose levels.[9]
Requirements
The Food and Nutrition Board (FNB) of the U.S. Institute of Medicine set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002. For isoleucine, for adults 19 years and older, 19 mg/kg body weight/day.[10]
Nutritional sources
Even though this amino acid is not produced in animals, it is stored in high quantities. Foods that have high amounts of isoleucine include eggs, soy protein, seaweed, turkey, chicken, lamb, cheese, and fish.[11]
Isomers
| Forms of Isoleucine | |||||||
|---|---|---|---|---|---|---|---|
| Common name: | isoleucine | d-isoleucine | l-isoleucine | dl-isoleucine | d-alloisoleucine | l-alloisoleucine | dl-alloisoleucine |
| Synonyms: | (R)-Isoleucine | L(+)-Isoleucine | (R*,R*)-isoleucine | alloisoleucine | |||
| PubChem: | CID 791 from PubChem | CID 94206 from PubChem | CID 6306 from PubChem | CID 76551 from PubChem | |||
| EINECS number: | |||||||
| CAS number: | 443-79-8 | 319-78-8 | 73-32-5 | 1509-35-9 | 1509-34-8 | 3107-04-8 | |
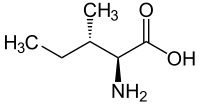 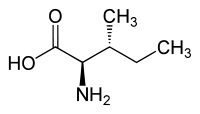 |
| l-isoleucine (2S,3S) and d-isoleucine (2R,3R) |
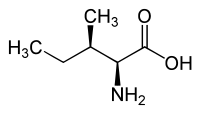 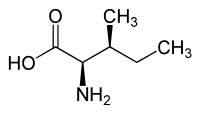 |
| l-alloisoleucine (2S,3R) and d-alloisoleucine (2R,3S) |
Synthesis
Isoleucine can be synthesized in a multistep procedure starting from 2-bromobutane and diethylmalonate.[12] Synthetic isoleucine was originally reported in 1905 by French chemist Louis Bouveault.[13]
German chemist Felix Ehrlich discovered isoleucine in hemoglobin in 1903.
References
- "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
- Kisumi M, Komatsubara S, Chibata I (July 1977). "Pathway for isoleucine formation form pyruvate by leucine biosynthetic enzymes in leucine-accumulating isoleucine revertants of Serratia marcescens". Journal of Biochemistry. 82 (1): 95–103. PMID 142769.
- "Maple Syrup Urine Disease (MSUD)". learn.genetics.utah.edu. Archived from the original on 2015-12-10. Retrieved 2015-12-08.
- Nelson DL, Cox MM (2000). Lehninger, Principles of Biochemistry (3rd ed.). New York: Worth Publishing. ISBN 1-57259-153-6.
- Cole JT. "Chapter 2: Metabolism of BCAAs" (PDF). In Rajendram R, Preedy VR, Patel VB (eds.). Branched Chain Amino Acids in Clinical Nutrition. 1. ISBN 978-1-4939-1923-9.
- Lynch CJ, Adams SH (December 2014). "Branched-chain amino acids in metabolic signalling and insulin resistance". Nature Reviews. Endocrinology. 10 (12): 723–36. doi:10.1038/nrendo.2014.171. PMC 4424797. PMID 25287287.
- Xiao F, Yu J, Guo Y, Deng J, Li K, Du Y, Chen S, Zhu J, Sheng H, Guo F (June 2014). "Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice". Metabolism. 63 (6): 841–50. doi:10.1016/j.metabol.2014.03.006. PMID 24684822.
- Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, et al. (December 2017). "Restoration of metabolic health by decreased consumption of branched-chain amino acids". The Journal of Physiology. doi:10.1113/JP275075. PMC 5813603. PMID 29266268.
- Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, et al. (July 2016). "Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health". Cell Reports. 16 (2): 520–530. doi:10.1016/j.celrep.2016.05.092. PMC 4947548. PMID 27346343.
- Institute of Medicine (2002). "Protein and Amino Acids". Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. pp. 589–768.
- "Foods highest in Isoleucine". Self Nutrition Data. Condé Nast.
List is in order of highest to lowest of per 200 Calorie serving of the food, not volume or weight.
- Marvel, C. S. (1941). "dl-Isoleucine (α-Amino-β-methylvaleric acid)". Organic Syntheses. 21: 60. doi:10.15227/orgsyn.021.0060.; Collective Volume, 3, p. 495
- Bouveault L, Locquin R (1905). "Action du sodium sur les éthers des acides monobasiques à fonction simple de la série grasse" [Effect of sodium on the ethers of single-function monobasic acids of the fatty series]. Compt. Rend. (in French). 140: 1593–1595.