Intrauterine growth restriction
Intrauterine growth restriction (IUGR) refers to poor growth of a fetus while in the mother's womb during pregnancy. The causes can be many, but most often involve poor maternal nutrition or lack of adequate oxygen supply to the fetus.
| Intrauterine growth restriction | |
|---|---|
| Other names | Fetal growth restriction (FGR),[1][2] intrauterine growth retardation,[3][4] |
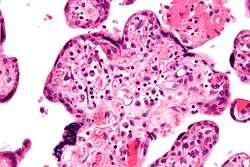 | |
| Micrograph of villitis of unknown etiology, a placental pathology associated with IUGR. H&E stain. | |
| Specialty | Pediatrics, obstetrics |
At least 60% of the 4 million neonatal deaths that occur worldwide every year are associated with low birth weight (LBW), caused by intrauterine growth restriction (IUGR), preterm delivery, and genetic abnormalities,[5] demonstrating that under-nutrition is already a leading health problem at birth.
Intrauterine growth restriction can result in a baby being small for gestational age (SGA), which is most commonly defined as a weight below the 10th percentile for the gestational age.[6] At the end of pregnancy, it can result in a low birth weight.
Types
There are 2 major categories of IUGR: pseudo IUGR and True IUGR
With pseudo IUGR, the fetus has birth weight below tenth centiles needed in average for the corresponding GA but have normal ponderal index, subcutaneous fat deposition, body proportion.Here IUGR occurred due to uneventful intra uterine course and can be rectified by proper postnatal care and nutrition. Such babies are also called as small baby for the gestational age.
True IUGR:here IUGR occurred due to pathological conditions which may be either fetal or maternal in origin. In addition to low body weight they have abnormal ponderal index, body disproportion, low subcutaneous fat deposition. It is of two types, symmetrical and asymmetrical.[7][8] Some conditions are associated with both symmetrical and asymmetrical growth restriction.
Asymmetrical
In asymmetrical IUGR, there is restriction of weight followed by length. The head continues to grow at normal or near-normal rates (head/brain sparing). A lack of subcutaneous fat leads to a thin and small body out of proportion with the liver. Normally at birth the brain of the fetus is 3 times the weight of its liver. In IUGR, it becomes 5-6 times. In these cases, the embryo/fetus has grown normally for the first two trimesters but encounters difficulties in the third, sometimes secondary to complications such as pre-eclampsia. Other symptoms than the disproportion include dry, peeling skin and an overly-thin umbilical cord. The baby is at increased risk of hypoxia and hypoglycaemia. This type of IUGR is most commonly caused by extrinsic factors that affect the fetus at later gestational ages. Specific causes include:
- Chronic high blood pressure
- Severe malnutrition
- Genetic mutations, Ehlers–Danlos syndrome
Symmetrical
Symmetrical IUGR is commonly known as global growth restriction, and indicates that the fetus has developed slowly throughout the duration of the pregnancy and was thus affected from a very early stage. The head circumference of such a newborn is in proportion to the rest of the body. Since most neurons are developed by the 18th week of gestation, the fetus with symmetrical IUGR is more likely to have permanent neurological sequelae. Common causes include:
- Early intrauterine infections, such as cytomegalovirus, rubella or toxoplasmosis
- Chromosomal abnormalities
- Anemia
- Maternal substance abuse (prenatal alcohol use can result in Fetal alcohol syndrome)
Causes
Maternal
- pre-pregnancy weight and nutritional status
- poor weight gain during pregnancy
- poor nutrition
- anemia
- alcohol and/or drug use
- maternal smoking
- recent pregnancy
- pre-gestational diabetes
- gestational diabetes
- pulmonary disease
- cardiovascular disease
- renal disease
- hypertension
- celiac disease increases the risk of intrauterine growth restriction by an odds ratio of approximately 2.48[9]
- blood clotting disorder/disease (Ex. Factor V Leiden)
Uteroplacental
- preeclampsia
- multiple gestation
- uterine malformations
- Placental insufficiency
Fetal
- chromosomal abnormalities
- Vertically transmitted infections
- Erythroblastosis fetalis
- Congenital abnormalities
Pathophysiology
If the cause of IUGR is extrinsic to the fetus (maternal or uteroplacental), transfer of oxygen and nutrients to the fetus is decreased. This causes a reduction in the fetus’ stores of glycogen and lipids. This often leads to hypoglycemia at birth. Polycythemia can occur secondary to increased erythropoietin production caused by the chronic hypoxemia. Hypothermia, thrombocytopenia, leukopenia, hypocalcemia, and pulmonary hemorrhage are often results of IUGR.
If the cause of IUGR is intrinsic to the fetus, growth is restricted due to genetic factors or as a sequela of infection.
IUGR is associated with a wide range of short- and long-term neurodevelopmental disorders
Cerebral changes
white matter effects – In postpartum studies of infants, it was shown that there was a decrease of the fractal dimension of the white matter in IUGR infants at one year corrected age. This was compared to at term and preterm infants at one year adjusted corrected age.
grey matter effects – Grey matter was also shown to be decreased in infants with IUGR at one year corrected age.
Neural circuitry
Children with IUGR are often found to exhibit brain reorganization including neural circuitry.[10] Reorganization has been linked to learning and memory differences between children born at term and those born with IUGR.[11]
Studies have shown that children born with IUGR had lower IQ. They also exhibit other deficits that point to [frontal lobe] dysfunction.
IUGR infants with brain-sparing show accelerated maturation of the hippocampus which is responsible for memory.[12] This accelerated maturation can often lead to uncharacteristic development that may compromise other networks and lead to memory and learning deficiencies.
Management
Bed rest has not been found to improve outcomes and therefore is not typically recommended.[13]
Mothers whose fetus is diagnosed with intrauterine growth restriction by ultrasound can use management strategies based on monitoring and delivery methods. One of these monitoring techniques is an umbilical artery Doppler. This method has been shown to decrease risk of morbidity and mortality before and after parturition among IUGR patients.[14]
Time of delivery is also a management strategy and is based on parameters collected from the umbilical artery doppler. Some of these include: pulsatility index, resistance index, and end-diastolic velocities, which are measurements of the fetal circulation.[14]
L-arginine has tentative evidence of benefit in reducing intrauterine growth restriction.[15]
Outcomes
By definition, IUGR affects 10% of pregnancies, however when corrected for several factors such as low maternal weight it is estimated only around 3% of pregnancies are affected by true IUGR. 20% of stillborn infants have IUGR. Perinatal mortality rates are 4-8 times higher for infants with IUGR, and morbidity is present in 50% of surviving infants.
According to the theory of thrifty phenotype, intrauterine growth restriction triggers epigenetic responses in the fetus that are otherwise activated in times of chronic food shortage. If the offspring actually develops in an environment rich in food it may be more prone to metabolic disorders, such as obesity and type II diabetes.[16]
Sheep
In sheep, intrauterine growth restriction can be caused by heat stress in early to mid pregnancy. The effect is attributed to reduced placental development causing reduced fetal growth.[17][18][19] Hormonal effects appear implicated in the reduced placental development.[19] Although early reduction of placental development is not accompanied by concurrent reduction of fetal growth;[17] it tends to limit fetal growth later in gestation. Normally, ovine placental mass increases until about day 70 of gestation,[20] but high demand on the placenta for fetal growth occurs later. (For example, research results suggest that a normal average singleton Suffolk x Targhee sheep fetus has a mass of about 0.15 kg at day 70, and growth rates of about 31 g/day at day 80, 129 g/day at day 120 and 199 g/day at day 140 of gestation, reaching a mass of about 6.21 kg at day 140, a few days before parturition.[21])
In adolescent ewes (i.e. ewe hoggets), overfeeding during pregnancy can also cause intrauterine growth restriction, by altering nutrient partitioning between dam and conceptus.[22][23] Fetal growth restriction in adolescent ewes overnourished during early to mid pregnancy is not avoided by switching to lower nutrient intake after day 90 of gestation; whereas such switching at day 50 does result in greater placental growth and enhanced pregnancy outcome.[23] Practical implications include the importance of estimating a threshold for "overnutrition" in management of pregnant ewe hoggets. In a study of Romney and Coopworth ewe hoggets bred to Perendale rams, feeding to approximate a conceptus-free live mass gain of 0.15 kg/day (i.e. in addition to conceptus mass), commencing 13 days after the midpoint of a synchronized breeding period, yielded no reduction in lamb birth mass, where compared with feeding treatments yielding conceptus-free live mass gains of about 0 and 0.075 kg/day.[24] In both of the above models of IUGR in sheep, the absolute magnitude of uterine blood flow is reduced.[23] Evidence of substantial reduction of placental glucose transport capacity has been observed in pregnant ewes that had been heat-stressed during placental development.[25][26]
See also
- Runt
- Interspecific pregnancy can cause this in animals
References
- "UpToDate".
- "Intrauterine Growth Restriction. IUGR information".
- Vandenbosche, Robert C.; Kirchner, Jeffrey T. (15 October 1998). "Intrauterine Growth Retardation". American Family Physician. 56 (6): 1384–1390. Retrieved 20 February 2016.
Intrauterine growth retardation (IUGR), which is defined as less than 10 percent of predicted fetal weight for gestational age, may result in significant fetal morbidity and mortality if not properly diagnosed. The condition is most commonly caused by inadequate maternal-fetal circulation, with a resultant decrease in fetal growth.
- White, Cynthia D. (16 November 2014). "Intrauterine growth restriction". MedlinePlus Medical Encyclopedia. Retrieved 21 February 2016.
Alternative Names: Intrauterine growth retardation; IUGR
- Lawn JE, Cousens S, Zupan J (2005). "4 million neonatal deaths: when? Where? Why?". The Lancet. 365 (9462): 891–900. doi:10.1016/s0140-6736(05)71048-5. PMID 15752534.
- Small for gestational age (SGA) at MedlinePlus. Update Date: 8/4/2009. Updated by: Linda J. Vorvick. Also reviewed by David Zieve.
- "Intrauterine Growth Restriction". Archived from the original on 2007-06-09. Retrieved 2007-11-28.
- Hunter, Stephen K.; Kennedy, Colleen M.; Peleg, David (August 1998). "Intrauterine Growth Restriction: Identification and Management - August 1998 - American Academy of Family Physicians". American Family Physician. 58 (2): 453–60, 466–7. PMID 9713399. Retrieved 2007-11-28.
- Saccone G, Berghella V, Sarno L, Maruotti GM, Cetin I, Greco L, Khashan AS, McCarthy F, Martinelli D, Fortunato F, Martinelli P (Oct 9, 2015). "Celiac disease and obstetric complications: a systematic review and metaanalysis". Am J Obstet Gynecol. 214 (2): 225–34. doi:10.1016/j.ajog.2015.09.080. PMID 26432464.
- Batalle D, Eixarch E, Figueras F, Muñoz-Moreno E, Bargallo N, Illa M, Acosta-Rojas R, Amat-Roldan I, Gratacos E (2012). "Altered small-world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome". NeuroImage. 60 (2): 1352–66. doi:10.1016/j.neuroimage.2012.01.059. PMID 22281673.
- Geva R, Eshel R, Leitner Y, Valevski AF, Harel S (2006). "Neuropsychological Outcome of Children With Intrauterine Growth Restriction: A 9-Year Prospective Study". Pediatrics. 118 (1): 91–100. doi:10.1542/peds.2005-2343. PMID 16818553.
- Black LS, deRegnier RA, Long J, Georgieff MK, Nelson CA (November 2004). "Electrographic imaging of recognition memory in 34-38 week gestation intrauterine growth restricted newborns". Experimental Neurology. 190 Suppl 1: S72–83. doi:10.1016/j.expneurol.2004.05.031. PMID 15498545.
- McCall, CA; Grimes, DA; Lyerly, AD (June 2013). ""Therapeutic" bed rest in pregnancy: unethical and unsupported by data". Obstetrics and Gynecology. 121 (6): 1305–8. doi:10.1097/AOG.0b013e318293f12f. PMID 23812466.
- Sharma D, Shastri S, Sharma P (2016). "Intrauterine Growth Restriction: Antenatal and Postnatal Aspects". Clinical Medicine Insights. Pediatrics. 10: 67–83. doi:10.4137/CMPed.S40070. PMC 4946587. PMID 27441006.
- Chen, J; Gong, X; Chen, P; Luo, K; Zhang, X (16 August 2016). "Effect of L-arginine and sildenafil citrate on intrauterine growth restriction fetuses: a meta-analysis". BMC Pregnancy and Childbirth. 16: 225. doi:10.1186/s12884-016-1009-6. PMC 4986189. PMID 27528012.
- Barker, D. J. P., ed. (1992). Fetal and infant origins of adult disease. London: British Medical Journal. ISBN 978-0-7279-0743-1.
- Vatnick I, Ignotz G, McBride BW, Bell AW (September 1991). "Effect of heat stress on ovine placental growth in early pregnancy". Journal of Developmental Physiology. 16 (3): 163–6. PMID 1797923.
- Bell A. W.; McBride B. W.; Slepetis R.; Early R. J.; Currie W. B. (1989). "Chronic Heat Stress and Prenatal Development in Sheep: I. Conceptus Growth and Maternal Plasma Hormones and Metabolites". Journal of Animal Science. 67 (12): 3289–3299. doi:10.2527/jas1989.67123289x. PMID 2613577.
- Regnault TR, Orbus RJ, Battaglia FC, Wilkening RB, Anthony RV (September 1999). "Altered arterial concentrations of placental hormones during maximal placental growth in a model of placental insufficiency". The Journal of Endocrinology. 162 (3): 433–42. doi:10.1677/joe.0.1620433. PMID 10467235.
- Ehrhardt RA, Bell AW (December 1995). "Growth and metabolism of the ovine placenta during mid-gestation". Placenta. 16 (8): 727–41. doi:10.1016/0143-4004(95)90016-0. PMID 8710803.
- Rattray PV, Garrett WN, East NE, Hinman N (March 1974). "Growth, development and composition of the ovine conceptus and mammary gland during pregnancy". Journal of Animal Science. 38 (3): 613–26. doi:10.2527/jas1974.383613x. PMID 4819552.
- Wallace J. M. (2000). "Nutrient partitioning during pregnancy: adverse gestational outcome in overnourished adolescent dams". Proc. Nutr. Soc. 59 (1): 107–117. doi:10.1017/s0029665100000136. PMID 10828180.
- Wallace J. M.; Regnault T. R. H.; Limesand S. W.; Hay Jr.; Anthony R. V. (2005). "Investigating the causes of low birth weights in contrasting ovine paradigms". J. Physiol. 565 (Pt 1): 19–26. doi:10.1113/jphysiol.2004.082032. PMC 1464509. PMID 15774527.
- Morris ST, Kenyon PR, West DM (2010). "Effect of hogget nutrition in pregnancy on lamb birthweight and survival to weaning". New Zealand Journal of Agricultural Research. 48 (2): 165–175. doi:10.1080/00288233.2005.9513647. ISSN 0028-8233.
- Bell AW, Wilkening RB, Meschia G (February 1987). "Some aspects of placental function in chronically heat-stressed ewes". Journal of Developmental Physiology. 9 (1): 17–29. PMID 3559063.
- Thureen PJ, Trembler KA, Meschia G, Makowski EL, Wilkening RB (September 1992). "Placental glucose transport in heat-induced fetal growth retardation". The American Journal of Physiology. 263 (3 Pt 2): R578–85. doi:10.1152/ajpregu.1992.263.3.R578. PMID 1415644.
External links
| Classification | |
|---|---|
| External resources |