Halloysite
Halloysite is an aluminosilicate clay mineral with the empirical formula Al2Si2O5(OH)4. Its main constituents are oxygen (55.78%), silicon (21.76%), aluminium (20.90%), and hydrogen (1.56%). Halloysite typically forms by hydrothermal alteration of alumino-silicate minerals.[4] It can occur intermixed with dickite, kaolinite, montmorillonite and other clay minerals. X-ray diffraction studies are required for positive identification. It was first described in 1826 and named after the Belgian geologist Omalius d'Halloy.
| Halloysite | |
|---|---|
 | |
| General | |
| Category | Phyllosilicates Kaolinite-serpentine group |
| Formula (repeating unit) | Al2Si2O5(OH)4 |
| Strunz classification | 9.ED.10 |
| Crystal system | Monoclinic |
| Crystal class | Domatic (m) (same H-M symbol) |
| Space group | Cc |
| Unit cell | a = 5.14, b = 8.9, c = 7.214 [Å]; β = 99.7°; Z = 1 |
| Identification | |
| Color | White; grey, green, blue, yellow, red from included impurities. |
| Crystal habit | Spherical clusters, massive |
| Cleavage | Probable on {001} |
| Fracture | Conchoidal |
| Mohs scale hardness | 2-2.5 |
| Luster | Pearly, waxy, or dull |
| Diaphaneity | Semitransparent |
| Specific gravity | 2-2.65 |
| Optical properties | Biaxial |
| Refractive index | nα = 1.553–1.565 nβ = 1.559–1.569 nγ = 1.560–1.570 |
| Birefringence | δ = 0.007 |
| References | [1][2][3] |
Occurrence

The formation of halloysite is due to hydrothermal alteration, and it is often found near carbonate rocks. For example, halloysite samples found in Wagon Wheel Gap, Colorado, United States are suspected to be the weathering product of rhyolite by downward moving waters.[4] In general the formation of clay minerals is highly favoured in tropical and sub-tropical climates due to the immense amounts of water flow. Halloysite has also been found overlaying basaltic rock, showing no gradual changes from rock to mineral formation.[6] Halloysite occurs primarily in recently exposed volcanic-derived soils, but it also forms from primary minerals in tropical soils or pre-glacially weathered materials.[7] Igneous rocks, especially glassy basaltic rocks are more susceptible to weathering and alteration forming halloysite.
Often as is the case with halloysite found in Juab County, Utah, United States the clay is found in close association with goethite and limonite and often interspersed with alunite. Feldspars are also subject to decomposition by water saturated with carbon dioxide. When feldspar occurs near the surface of lava flows, the CO2 concentration is high, and reaction rates are rapid. With increasing depth, the leaching solutions become saturated with silica, aluminium, sodium, and calcium. Once the solutions are depleted of CO2 they precipitate as secondary minerals. The decomposition is dependent on the flow of water. In the case that halloysite is formed from plagioclase it will not pass through intermediate stages.[4]
One of the largest halloysite deposits in the world is Dunino, near Legnica in Poland. It has reserves estimated at 10 million tons of material. This halloysite is characterized by layered-tubular and platy structure.[8]
Structure
Halloysite naturally occurs as small cylinders (nanotubes) that have a wall thickness of 10–15 atomic alumosilicate sheets, an outer diameter of 50–60 nm, an inner diameter of 12–15 nm, and a length of 0.5–10 μm. Their outer surface is mostly composed of SiO2 and the inner surface of Al2O3, and hence those surfaces are oppositely charged.[5][9] Two common forms are found. When hydrated, the clay exhibits a 1 nm spacing of the layers, and when dehydrated (meta-halloysite), the spacing is 0.7 nm. The cation exchange capacity depends on the amount of hydration, as 2H2O has 5–10 meq/100 g, while 4H2O has 40–50 meq/100g.[10] Endellite is the alternative name for the Al2Si2O5(OH)4·2(H2O) structure.[10][11]
Owing to the layered structure of the halloysite, it has a large specific surface area, which can reach 117 m2/g.[12]
Applications
Highly pure halloysite is mined from a rhyolite occurrence in New Zealand. Its uses include porcelain and bone china.[13][14][15][16]
Halloysite is an efficient adsorbent both for cations and anions. It has also been used as a petroleum cracking catalyst, and Exxon has developed a cracking catalyst based on synthetic halloysite in the 1970s.[17] Owing to its structure, halloysite can be used as filler in either natural or modified forms in nanocomposites. Halloysite nanotube can be intercalated with catalytic metal nanoparticles made of silver, ruthenium, rhodium, platinum or cobalt, thereby serving as a catalyst support.[5]
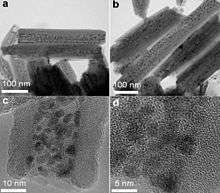
Besides supporting nanoparticles, halloysite nanotubes can also be used as a template to produce round well-dispersed nanoparticles (NPs). For example, bismuth and bismuth subcarbonate NPs with controlled size (~7 nm) were synthesized in water. Importantly, when halloysite was not used, large nanoplates instead of round spheres are obtained.[18]
References
- Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C., eds. (1995). "Halloysite" (PDF). Handbook of Mineralogy. Vol. II, 2003 Silica, Silicates. Chantilly, VA, US: Mineralogical Society of America. ISBN 978-0962209710.
- "Halloysite: Halloysite mineral information and data". mindat.org.
- Barthelmy, Dave. "Halloysite Mineral Data". webmineral.com.
- Kerr, Paul F. (1952). "Formation and occurrence of clay minerals". Clays and Clay Minerals. 1: 19–32. Bibcode:1952CCM.....1...19K. doi:10.1346/CCMN.1952.0010104.
- Vinokurov, Vladimir A.; Stavitskaya, Anna V.; Chudakov, Yaroslav A.; Ivanov, Evgenii V.; Shrestha, Lok Kumar; Ariga, Katsuhiko; Darrat, Yusuf A.; Lvov, Yuri M. (2017). "Formation of metal clusters in halloysite clay nanotubes". Science and Technology of Advanced Materials. 18 (1): 147–151. Bibcode:2017STAdM..18..147V. doi:10.1080/14686996.2016.1278352. PMC 5402758. PMID 28458738.
- Papke, Keith G. (1971). "Halloysite Deposits in the terraced Hills Washoe County, Nevada". Clays and Clay Minerals. 19 (2): 71–74. Bibcode:1971CCM....19...71P. doi:10.1346/CCMN.1971.0190202.
- Wilson M. J. (1999). "The Origin and Formation of Clay Minerals in Soils: Past Present and Future Perspectives". Clay Minerals. 34 (1): 7–25. Bibcode:1999ClMin..34....7W. doi:10.1180/000985599545957.
- Sakiewicz, P.; Lutynski, M.; Soltys, J.; Pytlinski, A. (2016). "Purification of Halloysite by Magnetic Separation". Physicochemical Problems of Mineral Processing. 52 (2): 991–1001. doi:10.5277/ppmp160236.
- Brindley, George W. (1952). "Structural mineralogy of clays". Clays and Clay Minerals. 1: 33–43. Bibcode:1952CCM.....1...33B. doi:10.1346/CCMN.1952.0010105.
- Carroll, Dorothy (1959). "Ion exchange in clays and other minerals". Geological Society of America Bulletin. 70 (6): 749‐780. Bibcode:1959GSAB...70..749C. doi:10.1130/0016-7606(1959)70[749:IEICAO]2.0.CO;2.
- Endellite. Webminerals
- Yang, Y. Zhang, and J. Ouyang (2016). "Physicochemical Properties of Halloysite". Nanosized Tubular Clay Minerals - Halloysite and Imogolite. Developments in Clay Science. 7. pp. 67–91. doi:10.1016/B978-0-08-100293-3.00004-2. ISBN 9780081002933.CS1 maint: multiple names: authors list (link)
- CASE STUDY: Halloysite Clay. minerals.co.nz
- Murray, H. H.; Harvey, C.; Smith, J. M. (1 February 1977). "Mineralogy and geology of the Maungaparerua halloysite deposit in New Zealand". Clays and Clay Minerals. 25 (1): 1–5. Bibcode:1977CCM....25....1M. doi:10.1346/CCMN.1977.0250101.
- "Common molecules sample 50642". Reciprocal Net.
- Lyday, Travis Q. (2002) The Mineral Industry of New Zealand. minerals.usgs.gov
- Robson, Harry E., Exxon Research & Engineering Co. (1976) "Synthetic halloysites as hydrocarbon conversion catalysts" U.S. Patent 4,098,676
- Ortiz-Quiñonez, J.L.; Vega-Verduga, C; Díaz, D; Zumeta-Dubé, I (June 13, 2018). "Transformation of Bismuth and β‑Bi2O3 Nanoparticles into (BiO)2CO3 and (BiO)4(OH)2CO3 by Capturing CO2: The Role of Halloysite Nanotubes and "Sunlight" on the Crystal Shape and Size". Crystal Growth & Design. 18 (8): 4334−4346. doi:10.1021/acs.cgd.8b00177.