HDAC7
Histone deacetylase 7 is an enzyme that in humans is encoded by the HDAC7 gene.[5][6][7]
Function
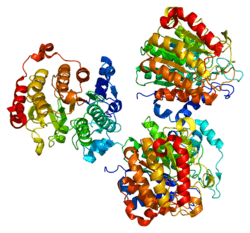
Histones play a critical role in transcriptional regulation, cell cycle progression, and developmental events. Histone acetylation/deacetylation alters chromosome structure and affects transcription factor access to DNA. The protein encoded by this gene has sequence homology to members of the histone deacetylase family. This gene is orthologous to mouse HDAC7 gene whose protein promotes repression mediated via transcriptional corepressor SMRT. Multiple alternatively spliced transcript variants encoding several isoforms have been found for this gene.[7] HDAC7 has both structural and functional similarity to HDACs 4, 5, and 9, as these four HDACs make up the Class IIa of HDACs in higher eukaryotes. Class IIa HDACs are phosphorylated by calcium/calmodulin dependent-kindase (CaMK) and protein kinase D (PKD) in response to kinase-dependent signaling. HDAC7 possesses little intrinsic deacetylase activity and therefore requires association with the class I HDAC, HDAC3 in order to suppress gene expression. It has been demonstrated through crystal structures of the human HDAC7 that the catalytic domain of HDAC7 has an additional class IIa HDAC-specific zinc binding motif adjacent to the active site.[8] This is most likely to allow for substrate recognition and protein-protein interactions that are necessary for class IIa HDAC enzymes.
Alternative functions
Although HDAC7 has shown to have little intrinsic deacetylase activity, studies have shown that HDAC7 may have various alternative functions related to development, proliferation, and inflammation.
One study showed that HDAC7 suppresses proliferation and β-catenin activity in chondrocytes. This was shown by knocking out HDAC7 in mice, which then resulted in increased levels of the cell cycle regulator, cyclin D3; decreased levels of the tumor suppressor, p21; and increased levels of active beta-catenin. Since each of these contribute to regulating cell proliferation, deletion of HDAC7 increased chondrocyte proliferation. This study also showed that signaling via the insulin/Insulin-like growth factor 1 receptor led to increased levels of HDAC7 in the cytosol than the nucleus and increased levels of active β-catenin, indicating that HDAC7 associates with β-catenin. During chondrogenesis, HDAC7 is translocated to the cytosol to be degraded, indicating that generally HDAC7 represses β-catenin activity in chondrocytes.[9]
Another study supported the conclusion that HDAC7 and β-catenin associate together by demonstrating that HDAC7 controls endothelial cell growth through modulation of β-catenin. This was shown in the opposite way from the previous study, in that HDAC7 was overexpressed rather than removed. They found that overexpression of HDAC7 prevented nuclear translocation of β-catenin which then coincided with downregulation of the cell cycle regulator, cyclin D1. Overall, this study demonstrated that HDAC7 once again interacts with β-catenin to keep endothelial cells in a low proliferation stage.[10]
Not only does HDAC7 play a role in the proliferation of cell growth in chondrocytes and endothelial cells, but it has also been demonstrated that HDAC7 is a crucial player in cancer cell proliferation through a study that showed mechanistic insight into the contribution of HDAC7 to tumor progression. This study showed that knockdown of HDAC7 resulted in significant cell arrest between the G(1) and S phases of the cell cycle. Subsequently, HDAC7 knockdown suppressed c-Myc expression which in turn blocked cell cycle progression. Through chromatin immunoprecipitation assays, it was shown that HDAC7 directly binds with the c-Myc gene and therefore HDAC7 silencing decreased c-Myc mRNA levels.[11]
Outside of proliferation, an additional study demonstrated that HDAC7 promotes inflammatory responses in macrophages. This was shown by overexpression of HDAC7 in inflammatory macrophages in mice. This overexpression promoted lipopolysaccharide (LPS)-inducible expression of HDAC-dependent genes via a HIF-1alpha-dependent mechanism. This demonstrated that HDAC7 may be a viable target for developing new anti-inflammatory drugs.[12]
Interactions
HDAC7A has been shown to interact with:
See also
References
- GRCh38: Ensembl release 89: ENSG00000061273 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000022475 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Marks PA, Richon VM, Rifkind RA (August 2000). "Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells". Journal of the National Cancer Institute. 92 (15): 1210–6. doi:10.1093/jnci/92.15.1210. PMID 10922406.
- Kao HY, Downes M, Ordentlich P, Evans RM (January 2000). "Isolation of a novel histone deacetylase reveals that class I and class II deacetylases promote SMRT-mediated repression". Genes & Development. 14 (1): 55–66. doi:10.1101/gad.14.1.55 (inactive 2020-05-21). PMC 316336. PMID 10640276.
- "Entrez Gene: HDAC7A histone deacetylase 7A".
- Schuetz A, Min J, Allali-Hassani A, Schapira M, Shuen M, Loppnau P, Mazitschek R, Kwiatkowski NP, Lewis TA, Maglathin RL, McLean TH, Bochkarev A, Plotnikov AN, Vedadi M, Arrowsmith CH (April 2008). "Human HDAC7 harbors a class IIa histone deacetylase-specific zinc binding motif and cryptic deacetylase activity". The Journal of Biological Chemistry. 283 (17): 11355–63. doi:10.1074/jbc.M707362200. PMC 2431080. PMID 18285338.
- Bradley EW, Carpio LR, Olson EN, Westendorf JJ (January 2015). "Histone deacetylase 7 (Hdac7) suppresses chondrocyte proliferation and β-catenin activity during endochondral ossification". The Journal of Biological Chemistry. 290 (1): 118–26. doi:10.1074/jbc.M114.596247. PMC 4281714. PMID 25389289.
- Margariti A, Zampetaki A, Xiao Q, Zhou B, Karamariti E, Martin D, Yin X, Mayr M, Li H, Zhang Z, De Falco E, Hu Y, Cockerill G, Xu Q, Zeng L (April 2010). "Histone deacetylase 7 controls endothelial cell growth through modulation of beta-catenin". Circulation Research. 106 (7): 1202–11. doi:10.1161/CIRCRESAHA.109.213165. PMID 20224040.
- Zhu C, Chen Q, Xie Z, Ai J, Tong L, Ding J, Geng M (March 2011). "The role of histone deacetylase 7 (HDAC7) in cancer cell proliferation: regulation on c-Myc". Journal of Molecular Medicine. 89 (3): 279–89. doi:10.1007/s00109-010-0701-7. PMID 21120446.
- Shakespear MR, Hohenhaus DM, Kelly GM, Kamal NA, Gupta P, Labzin LI, Schroder K, Garceau V, Barbero S, Iyer A, Hume DA, Reid RC, Irvine KM, Fairlie DP, Sweet MJ (2013). "Histone deacetylase 7 promotes Toll-like receptor 4-dependent proinflammatory gene expression in macrophages". The Journal of Biological Chemistry. 288 (35): 25362–74. doi:10.1074/jbc.M113.496281. PMC 3757200. PMID 23853092.
- Lemercier C, Brocard MP, Puvion-Dutilleul F, Kao HY, Albagli O, Khochbin S (June 2002). "Class II histone deacetylases are directly recruited by BCL6 transcriptional repressor" (PDF). The Journal of Biological Chemistry. 277 (24): 22045–52. doi:10.1074/jbc.M201736200. PMID 11929873.
- Lee HJ, Chun M, Kandror KV (May 2001). "Tip60 and HDAC7 interact with the endothelin receptor a and may be involved in downstream signaling". The Journal of Biological Chemistry. 276 (20): 16597–600. doi:10.1074/jbc.C000909200. PMID 11262386.
- Fischle W, Dequiedt F, Fillion M, Hendzel MJ, Voelter W, Verdin E (September 2001). "Human HDAC7 histone deacetylase activity is associated with HDAC3 in vivo". The Journal of Biological Chemistry. 276 (38): 35826–35. doi:10.1074/jbc.M104935200. PMID 11466315.
- Xiao H, Chung J, Kao HY, Yang YC (March 2003). "Tip60 is a co-repressor for STAT3". The Journal of Biological Chemistry. 278 (13): 11197–204. doi:10.1074/jbc.M210816200. PMID 12551922.
- Koipally J, Georgopoulos K (August 2002). "A molecular dissection of the repression circuitry of Ikaros". The Journal of Biological Chemistry. 277 (31): 27697–705. doi:10.1074/jbc.M201694200. PMID 12015313.
Further reading
- Verdin E, Dequiedt F, Kasler HG (May 2003). "Class II histone deacetylases: versatile regulators". Trends in Genetics. 19 (5): 286–93. doi:10.1016/S0168-9525(03)00073-8. PMID 12711221.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Lee HJ, Chun M, Kandror KV (May 2001). "Tip60 and HDAC7 interact with the endothelin receptor a and may be involved in downstream signaling". The Journal of Biological Chemistry. 276 (20): 16597–600. doi:10.1074/jbc.C000909200. PMID 11262386.
- Fischle W, Dequiedt F, Fillion M, Hendzel MJ, Voelter W, Verdin E (September 2001). "Human HDAC7 histone deacetylase activity is associated with HDAC3 in vivo". The Journal of Biological Chemistry. 276 (38): 35826–35. doi:10.1074/jbc.M104935200. PMID 11466315.
- Lemercier C, Brocard MP, Puvion-Dutilleul F, Kao HY, Albagli O, Khochbin S (June 2002). "Class II histone deacetylases are directly recruited by BCL6 transcriptional repressor" (PDF). The Journal of Biological Chemistry. 277 (24): 22045–52. doi:10.1074/jbc.M201736200. PMID 11929873.
- Bryant H, Farrell PJ (October 2002). "Signal Transduction and Transcription Factor Modification during Reactivation of Epstein-Barr Virus from Latency". Journal of Virology. 76 (20): 10290–8. doi:10.1128/JVI.76.20.10290-10298.2002. PMC 136559. PMID 12239305.
- Xiao H, Chung J, Kao HY, Yang YC (March 2003). "Tip60 is a co-repressor for STAT3". The Journal of Biological Chemistry. 278 (13): 11197–204. doi:10.1074/jbc.M210816200. PMID 12551922.
- Dequiedt F, Kasler H, Fischle W, Kiermer V, Weinstein M, Herndier BG, Verdin E (May 2003). "HDAC7, a thymus-specific class II histone deacetylase, regulates Nur77 transcription and TCR-mediated apoptosis". Immunity. 18 (5): 687–98. doi:10.1016/S1074-7613(03)00109-2. hdl:11858/00-001M-0000-002C-9FC8-8. PMID 12753745.
- Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM, Curtiss LK (October 2003). "Transcriptional repression of atherogenic inflammation: modulation by PPARdelta". Science. 302 (5644): 453–7. Bibcode:2003Sci...302..453L. doi:10.1126/science.1087344. PMID 12970571.
- Li X, Song S, Liu Y, Ko SH, Kao HY (August 2004). "Phosphorylation of the histone deacetylase 7 modulates its stability and association with 14-3-3 proteins". The Journal of Biological Chemistry. 279 (33): 34201–8. doi:10.1074/jbc.M405179200. PMID 15166223.
- Kato H, Tamamizu-Kato S, Shibasaki F (October 2004). "Histone deacetylase 7 associates with hypoxia-inducible factor 1alpha and increases transcriptional activity". The Journal of Biological Chemistry. 279 (40): 41966–74. doi:10.1074/jbc.M406320200. PMID 15280364.
- Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villén J, Li J, Cohn MA, Cantley LC, Gygi SP (August 2004). "Large-scale characterization of HeLa cell nuclear phosphoproteins". Proceedings of the National Academy of Sciences of the United States of America. 101 (33): 12130–5. Bibcode:2004PNAS..10112130B. doi:10.1073/pnas.0404720101. PMC 514446. PMID 15302935.
- Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S, Metalnikov P, O'Donnell P, Taylor P, Taylor L, Zougman A, Woodgett JR, Langeberg LK, Scott JD, Pawson T (August 2004). "Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization". Current Biology. 14 (16): 1436–50. doi:10.1016/j.cub.2004.07.051. PMID 15324660.
External links
- HDAC7A+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.