Graptolithina
Graptolithina is a subclass of the class Pterobranchia, the members of which are known as graptolites. These organisms are colonial animals known chiefly as fossils from the Middle Cambrian (Miaolingian, Wuliuan) through the Lower Carboniferous (Mississippian).[3] A possible early graptolite, Chaunograptus, is known from the Middle Cambrian.[1] One analysis suggests that the pterobranch Rhabdopleura represents extant graptolites.[2] Studies on the tubarium of fossil and living graptolites showed similarities in the basic fusellar construction and it is considered that the group most probably evolved from a Rhabdopleura-like ancestor.[4]
| Graptolites | |
|---|---|
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Hemichordata |
| Class: | Pterobranchia |
| Subclass: | Graptolithina |
| Orders | |
| |
The name graptolite comes from the Greek graptos meaning "written", and lithos meaning "rock", as many graptolite fossils resemble hieroglyphs written on the rock. Linnaeus originally regarded them as 'pictures resembling fossils' rather than true fossils, though later workers supposed them to be related to the hydrozoans; now they are widely recognized as hemichordates.[4]
Taxonomy
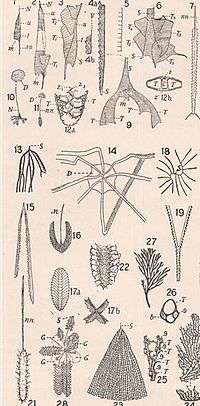
The name "graptolite" originates from the genus Graptolithus, which was used by Linnaeus in 1735 for inorganic mineralizations and incrustations which resembled actual fossils. In 1768, in the 12th volume of Systema Naturae, he included G. sagittarius and G. scalaris, respectively a possible plant fossil and a possible graptolite. In his 1751 Skånska Resa, he included a figure of a "fossil or graptolite of a strange kind" currently thought to be a type of Climacograptus (a genus of biserial graptolites). The term Graptolithina was established by Bronn in 1849 and later, Graptolithus was officially abandoned in 1954 by the ICZN.[5]
Since the 1970s, as a result of advances in electron microscopy, graptolites have generally been thought to be most closely allied to the pterobranchs, a rare group of modern marine animals belonging to the phylum Hemichordata.[6] Comparisons are drawn with the modern hemichordates Cephalodiscus and Rhabdopleura, and according to recent phylogenetic studies, rhabdopleurids are placed within the Graptolithina, nonetheless, they are considered an incertae sedis family.[3] On the other hand, Cephalodiscida is considered a sister subclass of Graptolithina. Some of the main differences between these two groups are that Cephalodiscida is not a colonial organism so there is not a common canal connecting all zooids, which also have several arms while Graptolithina zooids have a pair. Other differences include the type of early development, the gonads, the presence or absence of gill slits, and the size of the zooids. However, in the fossil record where mostly tubes are preserved, it is complicated to make the distinction between groups.
Graptolithina includes two main orders, Dendroidea (benthic graptolites) and Graptoloidea (planktic graptolites). The latter is the most diverse, including 5 suborders, where the most assorted is Axonophora. This group includes Diplograptids and Neograptids, groups that had a great development during the Ordovician.[3] Old taxonomic classifications consider the orders Dendroidea, Tuboidea, Camaroidea, Crustoidea, Stolonoidea, Graptoloidea, and Dithecoidea but new classifications embedded them into Graptoloidea at different taxonomic levels.
| Phylogeny of Pterobranchia[3] | |||||||||||||||
|
Subclass Graptolithina Bronn 1849
- Order ?†Camaroidea Kozlowski 1928 sensu Kozlowski 1949
- Family †Cysticamaridae Bulman 1955
- Order ?†Crustoidea Bulman 1970
- Family †Wimanicrustidae Bulman 1970
- Order ?†Dithecoidea Obut, 1960
- Family †Dithecodendridae Obut 1964
- Order ?†Tuboidea Kozlowski 1938 sensu Kozlowsk 1949
- Family †Cyclograptidae Bulman 1938
- Order Rhabdopleurida Fowler 1892 sensu Beklemishev 1951
- Family Rhabdopleuridae Harmer 1905
- Clade †Eugraptolithina Mitchell et al., 2013
- Order †Dendroidea Nicholson 1872
- Family †Acanthograptidae Bulman 1938
- Family †Dendrograptidae Römer 1897
- Family †Mastigograptidae Bates & Urbanek 2002
- Order †Graptoloidea Maletz, Carlucci and Mitchell 2009
- Suborder †Graptodendroidina Mu & Lin 1981
- Family †Anisograptidae Bulman 1950
- Suborder †Sinograpta Maletz et al. 2009
- Family †Abrograptidae Mu 1958
- Family †Sigmagraptidae Cooper & Fortey 1982
- Family †Sinograptidae Mu 1957
- Suborder †Dichograptina Lapworth 1873
- Family †Dichograptidae Lapworth 1873
- Family †Didymograptidae Mu 1950
- Family †Pterograptidae Mu 1950
- Family †Tetragraptidae (Frech 1897) Mu 1950
- Suborder †Glossograptina Jaanusson 1960
- Family †Isograptidae Harris 1933
- Family †Glossograptidae Lapworth 1873b
- Suborder †Axonophora Frech 1897
- Infraorder †Diplograptina Lapworth 1880e
- Family †Diplograptidae Lapworth 1873
- Family †Dicranograptidae Lapworth 1873
- Family †Climacograptidae Frech 1897 em. Přibyl 1948
- Family †Lasiograptidae Bulman 1955
- Infraorder †Neograptina Štorch et al. 2011
- Family †Neodiplograptidae Melchin et al. 2011
- Family †Normalograptidae Štorch & Serpagli 1993
- Family †Retiolitidae Lapworth 1873
- Family †Dimorphograptidae Elles & Wood 1908
- Family †Monograptidae Lapworth 1873
- Infraorder †Diplograptina Lapworth 1880e
- Suborder †Graptodendroidina Mu & Lin 1981
- Order †Dendroidea Nicholson 1872
Stratigraphy
Graptolites are common fossils and have a worldwide distribution. The preservation, quantity and gradual change over a geologic time scale of graptolites allow the fossils to be used to date strata of rocks throughout the world.[6] They are important index fossils for dating Palaeozoic rocks as they evolved rapidly with time and formed many different species. Geologists can divide the rocks of the Ordovician and Silurian periods into graptolite biozones; these are generally less than one million years in duration. A worldwide ice age at the end of the Ordovician eliminated most graptolites except the neograptines. Diversification from the neograptines that survived the Ordovician glaciation began around 2 million years later.[7]
Some of the greatest extinctions that affected the group were the Hirnantian in the Ordovician and the Lundgreni in the Silurian, where the graptolites populations were dramatically reduced. Particularly in the late Ordovician extinction, a recovery event known as the Great Ordovician Diversification Event or GOBE, influenced changes in the morphology of the colonies and thecae, giving rise to new groups like the planktic Graptoloidea.[4]
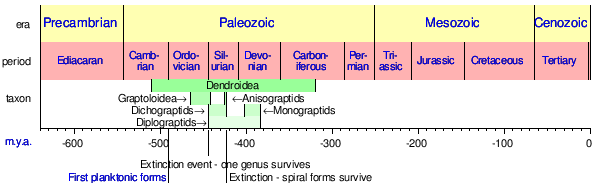 | |
| Ranges of Graptolite taxa. | |
Morphology
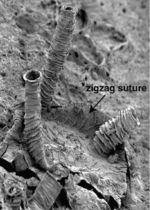
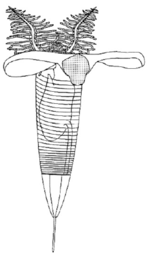
Each graptolite colony originates from an initial individual, called the sicular zooid, from which the subsequent zooids will develop; they are all interconnected by stolons. These zooids are housed within an organic tubular structure called a theca, rhabsodome, coenoecium or tubarium, which is secreted by the glands on the cephalic shield. The composition of the tubarium is not clearly known but different authors suggest it is made out of collagen or chitin. The tubarium has a variable number of branches or stipes and different arrangements of the theca, these features are important in the identification of graptolite fossils. In some colonies, there are two sizes of theca, the authoteca and the bitheca, and it has been suggested that this difference is due to sexual dimorphism.[4]
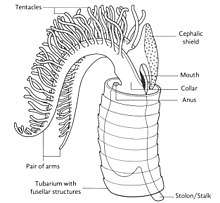
A mature zooid has three important regions, the preoral disc or cephalic shield, the collar and the trunk. In the collar, the mouth and anus (U-shaped digestive system) and arms are found; Graptholitina has a single pair of arms with several paired tentacles. As a nervous system, graptolites have a simple layer of fibers between the epidermis and the basal lamina, also have a collar ganglion that gives rise to several nerve branches, similar to the neural tube of chordates.[8] All this information was inferred by the extant Rhabdopleura, however, it is very likely that fossil zooids had the same morphology. An important feature in the tubarium is the fusellum, which looks like lines of growth along the tube observed as semicircular rings in a zig-zag pattern.
Most of the dendritic or bushy/fan-shaped organisms are classified as dendroid graptolites (order Dendroidea). They appear earlier in the fossil record during the Cambrian and were generally sessile animals. They lived attached to a hard substrate in the sea-floor, by their own weight as encrusting organisms or by an attachment disc. Graptolites with relatively few branches were derived from the dendroid graptolites at the beginning of the Ordovician period. This latter type (order Graptoloidea) were pelagic and planktonic, drifting freely on the surface of primitive seas. They were a successful and prolific group, being the most important animal members of the plankton until they partially died out in the early part of the Devonian period. The dendroid graptolites survived until the Carboniferous period.
Ecology
Graptolites were a major component of the early Paleozoic ecosystems, especially for the zooplankton because the most abundant and diverse species were planktonic. Graptolites were most likely suspension feeders and strained the water for food such as plankton.[9] Inferring by analogy with modern pterobranchs, they were able to migrate vertically through the water column for feeding efficiency and to avoid predators. With ecological models and studies of the facies, it was observed that, at least for Ordovician species, some groups of species are largely confined to the epipelagic and mesopelagic zone, from inshore to open ocean.[10] Living rhabdopleura have been found in deep waters in several regions of Europe and America but the distribution might be biased by sampling efforts; colonies are usually found as epibionts of shells.
Their locomotion was relative to the water mass in which they lived but the exact mechanisms (like turbulence, buoyancy, active swimming...) are not clear yet. The most likely option was rowing or swimming by undulatory motion with muscular appendages or with the feeding tentacles. However, in some species, the thecal aperture was probably so restricted that the appendages hypothesis is not feasible. On the other hand, buoyancy is not supported by any extra thecal tissue or gas build-up control mechanism, and active swimming requires a lot of energetic waste, which would rather be used for the tubarium construction.[10]
There are still many questions regarding graptolite locomotion but all these mechanisms are possible alternatives depending on the species and its habitat. For benthic species, that lived attached to the sediment or any other organism, this was not a problem; the zooids were able to move but restricted within the tubarium. Although this zooid movement is possible in both planktic and benthic species, it is limited by the stolon but is particularly useful for feeding. Using their arms and tentacles, which are close to the mouth, they filter the water to catch any particles of food.[10]
Life cycle
The study of the developmental biology of Graptholitina has been possible by the discovery of the species R. compacta and R. normani in shallow waters; it is assumed that graptolite fossils had a similar development as their extant representatives. The life cycle comprises two events, the ontogeny and the astogeny, where the main difference is whether the development is happening in the individual organism or in the modular growth of the colony.
The life cycle begins with a planktonic planula-like larva produced by sexual reproduction, which later becomes the sicular zooid who starts a colony. In Rhabdopleura, the colonies bear male and female zooids but fertilized eggs are incubated in the female tubarium, and stay there until they become larvae able to swim (after 4–7 days) to settle away to start a new colony. Each larva surrounds itself in a protective cocoon where the metamorphosis to the zooid takes place (7–10 days) and attaches with the posterior part of the body, where the stalk will eventually develop.[4]
The development is indirect and lecithotrophic, and the larvae are ciliated and pigmented, with a deep depression on the ventral side.[11][8] Astogeny happens when the colony grows through asexual reproduction from the tip of a permanent terminal zooid, behind which the new zooids are budded from the stalk, a type of budding called monopodial. It is possible that in graptolite fossils the terminal zooid was not permanent because the new zooids formed from the tip of latest one, in other words, sympodial budding. These new organisms break a hole in the tubarium wall and start secreting their own tube.[4]
Graptolites for evolutionary development

In recent years, living graptolites have been used as a hemichordate model for Evo-Devo studies, as have their sister group, the acorn worms. For example, graptolites are used to study asymmetry in hemichordates, especially because their gonads tend to be located randomly on one side. In Rhabdopleura normani, the testicle is located asymmetrically, and possibly other structures such as the oral lamella and the gonopore.[12] The significance of these discoveries is to understand the early vertebrate left-right asymmetry due to chordates are a sister group of hemichordates, and therefore, the asymmetry might be a feature that developed early in deuterostomes. Since the location of the structures is not strictly established, also in some enteropneusts, it is likely that asymmetrical states in hemichordates are not under a strong developmental or evolutionary constraint. The origin of this asymmetry, at least for the gonads, is possibly influenced by the direction of the basal coiling in the tubarium, by some intrinsic biological mechanisms in pterobranchs, or solely by environmental factors.[12]
Hedgehog (hh), a highly conserved gene implicated in neural developmental patterning, was analyzed in Hemichordates, taking Rhabdopleura as a pterobranch representative. It was found that hedgehog gene in pterobranchs is expressed in a different pattern compared to other hemichordates as the enteropneust Saccoglossus kowalevskii. An important conserved glycine–cysteine–phenylalanine (GCF) motif at the site of autocatalytic cleavage in hh genes, is altered in R. compacta by an insertion of the amino acid threonine (T) in the N-terminal, and in S. kowalesvskii there is a replacement of serine (S) for glycine (G). This mutation decreases the efficiency of the autoproteolytic cleavage and therefore, the signalling function of the protein. It is not clear how this unique mechanism occurred in evolution and the effects it has in the group, but, if it has persisted over millions of years, it implies a functional and genetic advantage.[13]
Preservation
Graptolite fossils are often found in shales and mudrocks where sea-bed fossils are rare, this type of rock having formed from sediment deposited in relatively deep water that had poor bottom circulation, was deficient in oxygen, and had no scavengers. The dead planktic graptolites, having sunk to the sea-floor, would eventually become entombed in the sediment and were thus well preserved.
These colonial animals are also found in limestones and cherts, but generally these rocks were deposited in conditions which were more favorable for bottom-dwelling life, including scavengers, and undoubtedly most graptolite remains deposited here were generally eaten by other animals.
Fossils are often found flattened along the bedding plane of the rocks in which they occur, though may be found in three dimensions when they are infilled by iron pyrite or some other minerals. They vary in shape, but are most commonly dendritic or branching (such as Dictyonema), saw-blade like, or "tuning fork" shaped (such as Didymograptus murchisoni). Their remains may be mistaken for fossil plants by the casual observer, as it has been the case for the first graptolite descriptions.
Graptolites are normally preserved as a black carbon film on the rock's surface or as light grey clay films in tectonically distorted rocks. The fossil can also appear stretched or distorted. This is due to the strata that the graptolite is within, being folded and compacted. They may be sometimes difficult to see, but by slanting the specimen to the light they reveal themselves as a shiny marking. Pyritized graptolite fossils are also found.
A well-known locality for graptolite fossils in Britain is Abereiddy Bay, Dyfed, Wales, where they occur in rocks from the Ordovician period. Sites in the Southern Uplands of Scotland, the Lake District and Welsh Borders also yield rich and well-preserved graptolite faunas. A famous graptolite location in Scotland is Dob's Linn with species from the boundary Ordovician-Silurian. However, since the group had a wide distribution, they are also abundantly found in several localities in the USA, Canada, Australia, Germany, China, among others.
See also
References
- Maletz, J. (2014). Hemichordata (Pterobranchia, Enteropneusta) and the fossil record. Palaeogeography, Palaeoclimatology, Palaeoecology, 398:16-27.
- Mitchell, C.E., Melchin, M.J., Cameron, C.B. & Maletz, J. (2013) Phylogenetic analysis reveals that Rhabdopleura is an extant graptolite. Lethaia, 46:34–56.
- Maletz, Jörg (2014). "The classification of the Pterobranchia (Cephalodiscida and Graptolithina)". Bulletin of Geosciences. 89 (3): 477–540. doi:10.3140/bull.geosci.1465. ISSN 1214-1119.
- Maletz, J. (2017) Graptolite Paleobiology. Willey-Blackwell, 336 p.
- Bulman, M. (1970) In Teichert, C. (ed.). Treatise on Invertebrate Paleontology. Part V. Graptolithina, with sections on Enteropneusta and Pterobranchia. (2nd Edition). Geological Society of America and University of Kansas Press, Boulder, Colorado and Lawrence, Kansas, XXXII + 163 pp.
- Fortey, Richard A. (1998). Life: A Natural History of the First Four Billion Years of Life on Earth. New York: Alfred A. Knopf. p. 129.
- Bapst, D., Bullock, P., Melchin, M., Sheets, D. & Mitchell, C. (2012) Graptoloid diversity and disparity became decoupled during the Ordovician mass extinction. Proceedings of the National Academy of Sciences, 109(9):3428-3433.
- Sato, A., Bishop, J. & Holland, P. (2008). Developmental Biology of Pterobranch Hemichordates: History and Perspectives. Genesis, 46:587-591.
- "Graptolites". samnoblemuseum.ou.edu. Retrieved 2018-12-28.
- Cooper, R., Rigby, S., Loydell, D. & Bates, D. (2012) Palaeoecology of the Graptoloidea. Earth-Science Reviews, 112(1):23-41.
- Röttinger, E. & Lowe, C. (2012) Evolutionary crossroads in developmental biology: hemichordates. Development, 139:2463-2475.
- Sato, A. & Holland, P. (2008). Asymmetry in a Pterobranch Hemichordate and the Evolution of Left-Right Patterning. Developmental Dynamics, 237:3634 –3639)
- Sato, A., White-Cooper, H., Doggett, K. & Holland, P. 2009. Degenerate evolution of the hedgehog gene in a hemichordate lineage. Proceedings of the National Academy of Sciences, 106(18):7491-7494.
External links
| Wikimedia Commons has media related to Graptolithina. |
- Classification of the Graptolithoidea - Graptolites and Pterobranchs
- Podcast on Graptolites by David Bapst - Palaeocast
- Graptolites gallery by Michael P. Klimetz - Graptolites
- What are Fossil Graptolites and why are they useful in geology? - Youtube
- Writing on the rocks - Stephen Hui Geological Museum
.jpg)
