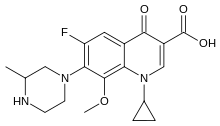
Gatifloxacin
Gatifloxacin (brand names Gatiflo, Tequin, and Zymar) is an antibiotic of the fourth-generation fluoroquinolone family,[1] that like other members of that family, inhibits the bacterial enzymes DNA gyrase and topoisomerase IV.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Gatiflo, Tequin, Zymar, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605012 |
| Routes of administration | Oral (discontinued), Intravenous (discontinued) ophthalmic |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 20% |
| Elimination half-life | 7 to 14 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.190.526 |
| Chemical and physical data | |
| Formula | C19H22FN3O4 |
| Molar mass | 375.400 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
It was patented in 1986 and approved for medical use in 1999.[2]
Side effects
A Canadian study published in the New England Journal of Medicine in March 2006 claimed that Tequin can have significant side effects including dysglycemia.[3] An editorial by Jerry Gurwitz in the same issue called for the Food and Drug Administration (FDA) to consider giving Tequin a black box warning.[4] This editorial followed distribution of a letter dated February 15 by Bristol-Myers Squibb to health care providers indicating action taken with the FDA to strengthen warnings for the medication.[5] Subsequently, Bristol-Myers Squibb reported it would stop manufacture of Tequin, end sales of the drug after existing stockpiles were exhausted, and return all rights to Kyorin.[6]
By contrast, ophthalmic gatifloxacin is generally well tolerated. The observed systemic concentration of the drug following oral administration of 400 mg gatifloxacin is approximately 800 times higher than that of the 0.5% gatifloxacin eye drop. Given as an eye drop, gatifloxacin has very low systemic exposure. Therefore, the systemic exposures resulting from the gatifloxacin ophthalmic solution are not likely to pose any risk for systemic toxicities.
Contraindications
Society and culture
Availability
Gatifloxacin is currently available in the US and Canada only as an ophthalmic solution.
In 2011, the Union Health and Family Welfare Ministry of India banned the manufacture, sale, and distribution of gatifloxacin because of its adverse side effects.[8]
In China, gatifloxacin is sold in tablet as well as in eye drop formulations.
Brand names
Bristol-Myers Squibb introduced gatifloxacin in 1999 under the proprietary name Tequin for the treatment of respiratory tract infections, having licensed the medication from Kyorin Pharmaceutical Company of Japan. Allergan produces it in eye-drop formulation under the names Zymar and Zymaxid. In many countries, gatifloxacin is also available as tablets and in various aqueous solutions for intravenous therapy.
References
- Burka JM, Bower KS, Vanroekel RC, Stutzman RD, Kuzmowych CP, Howard RS (July 2005). "The effect of fourth-generation fluoroquinolones gatifloxacin and moxifloxacin on epithelial healing following photorefractive keratectomy". Am. J. Ophthalmol. 140 (1): 83–7. doi:10.1016/j.ajo.2005.02.037. PMID 15953577.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 501. ISBN 9783527607495.
- Park-Wyllie, Laura Y.; David N. Juurlink; Alexander Kopp; Baiju R. Shah; Thérèse A. Stukel; Carmine Stumpo; Linda Dresser; Donald E. Low; Muhammad M. Mamdani (March 2006). "Outpatient Gatifloxacin Therapy and Dysglycemia in Older Adults". The New England Journal of Medicine. 354 (13): 1352–1361. doi:10.1056/NEJMoa055191. hdl:1807/16915. PMID 16510739. Note: publication date 30 March; available on-line 1 March
- Gurwitz, Jerry H. (March 2006). "Serious Adverse Drug Effects — Seeing the Trees through the Forest". The New England Journal of Medicine. 354 (13): 1413–1415. doi:10.1056/NEJMe068051. PMID 16510740.
- Lewis-Hall, Freda (February 15, 2006). "Dear Healthcare Provider" (PDF). Bristol-Myers Squibb. Retrieved May 1, 2006.
- Schmid, Randolph E. (May 1, 2006). "Drug Company Taking Tequin Off Market". Associated Press. Archived from the original on November 25, 2007. Retrieved 2006-05-01.
- Peggy Peck (2 May 2006). "Bristol-Myers Squibb Hangs No Sale Sign on Tequin". Med Page Today. Retrieved 24 February 2009.
- "Two drugs banned". The Hindu. Chennai, India. 19 March 2011.