Endosymbiont
An endosymbiont or endobiont[1] is any organism that lives within the body or cells of another organism most often, though not always, in a mutualistic relationship. (The term endosymbiosis is from the Greek: ἔνδον endon "within", σύν syn "together" and βίωσις biosis "living".) Examples are nitrogen-fixing bacteria (called rhizobia), which live in the root nodules of legumes; single-cell algae inside reef-building corals, and bacterial endosymbionts that provide essential nutrients to about 10–15% of insects.[2][3]
There are two types of symbiont transmissions. In horizontal transmission, each new generation acquires free living symbionts from the environment. An example is the nitrogen-fixing bacteria in certain plant roots. Vertical transmission takes place when the symbiont is transferred directly from parent to offspring. There is also a combination of these types, where symbionts are transferred vertically for some generation before a switch of host occurs and new symbionts are horizontally acquired from the environment. In vertical transmissions, the symbionts often have a reduced genome and are no longer able to survive on their own. As a result, the symbiont depends on the host, resulting in a highly intimate co-dependent relationship. For instance, pea aphid symbionts have lost genes for essential molecules, now relying on the host to supply them with nutrients. In return, the symbionts synthesize essential amino acids for the aphid host .[4] Other examples include Wigglesworthia nutritional symbionts of tse-tse flies, or in sponges.[5] When a symbiont reaches this stage, it begins to resemble a cellular organelle, similar to mitochondria or chloroplasts.
Many instances of endosymbiosis are obligate; that is, either the endosymbiont or the host cannot survive without the other, such as the gutless marine worms of the genus Riftia, which get nutrition from their endosymbiotic bacteria. The most common examples of obligate endosymbioses are mitochondria and chloroplasts. Some human parasites, e.g. Wuchereria bancrofti and Mansonella perstans, thrive in their intermediate insect hosts because of an obligate endosymbiosis with Wolbachia spp. They can both be eliminated from said hosts by treatments that target this bacterium. However, not all endosymbioses are obligate and some endosymbioses can be harmful to either of the organisms involved.
Two major types of organelle in eukaryotic cells, mitochondria and plastids such as chloroplasts, are considered to be bacterial endosymbionts.[6] This process is commonly referred to as symbiogenesis.
Symbiogenesis and organelles
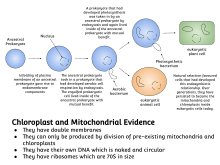
Symbiogenesis explains the origins of eukaryotes, whose cells contain two major kinds of organelle: mitochondria and chloroplasts. The theory proposes that these organelles evolved from certain types of bacteria that eukaryotic cells engulfed through phagocytosis. These cells and the bacteria trapped inside them entered an endosymbiotic relationship, meaning that the bacteria took up residence and began living exclusively within the eukaryotic cells.[7]
Numerous insect species have endosymbionts at different stages of symbiogenesis. A common theme of symbiogenesis involves the reduction of the genome to only essential genes for the host and symbiont collective genome.[8] A remarkable example of this is the fractionation of the Hodgkinia genome of Magicicada cicadas. Because the cicada life cycle takes years underground, natural selection on endosymbiont populations is relaxed for many bacterial generations. This allows the symbiont genomes to diversify within the host for years with only punctuated periods of selection when the cicadas reproduce. As a result, the ancestral Hodgkinia genome has split into three groups of primary endosymbiont, each encoding only a fraction of the essential genes for the symbiosis. The host now requires all three sub-groups of symbiont, each with degraded genomes lacking most essential genes for bacterial viability.[9]
Bacterial endosymbionts of invertebrates
The best-studied examples of endosymbiosis are known from invertebrates. These symbioses affect organisms with global impact, including symbiodinium of corals, or Wolbachia of insects. Many insect agricultural pests and human disease vectors have intimate relationships with primary endosymbionts.
Endosymbionts of insects
_-_with_key.png)
Scientists classify insect endosymbionts in two broad categories, 'Primary' and 'Secondary'. Primary endosymbionts (sometimes referred to as P-endosymbionts) have been associated with their insect hosts for many millions of years (from 10 to several hundred million years in some cases). They form obligate associations (see below), and display cospeciation with their insect hosts. Secondary endosymbionts exhibit a more recently developed association, are sometimes horizontally transferred between hosts, live in the hemolymph of the insects (not specialized bacteriocytes, see below), and are not obligate.[10]
Primary endosymbionts
Among primary endosymbionts of insects, the best-studied are the pea aphid (Acyrthosiphon pisum) and its endosymbiont Buchnera sp. APS,[11][12][4] the tsetse fly Glossina morsitans morsitans and its endosymbiont Wigglesworthia glossinidia brevipalpis and the endosymbiotic protists in lower termites. As with endosymbiosis in other insects, the symbiosis is obligate in that neither the bacteria nor the insect is viable without the other. Scientists have been unable to cultivate the bacteria in lab conditions outside of the insect. With special nutritionally-enhanced diets, the insects can survive, but are unhealthy, and at best survive only a few generations.
In some insect groups, these endosymbionts live in specialized insect cells called bacteriocytes (also called mycetocytes), and are maternally-transmitted, i.e. the mother transmits her endosymbionts to her offspring. In some cases, the bacteria are transmitted in the egg, as in Buchnera; in others like Wigglesworthia, they are transmitted via milk to the developing insect embryo. In termites, the endosymbionts reside within the hindguts and are transmitted through trophallaxis among colony members.
The primary endosymbionts are thought to help the host either by providing nutrients that the host cannot obtain itself or by metabolizing insect waste products into safer forms. For example, the putative primary role of Buchnera is to synthesize essential amino acids that the aphid cannot acquire from its natural diet of plant sap. Likewise, the primary role of Wigglesworthia, it is presumed, is to synthesize vitamins that the tsetse fly does not get from the blood that it eats. In lower termites, the endosymbiotic protists play a major role in the digestion of lignocellulosic materials that constitute a bulk of the termites' diet.
Bacteria benefit from the reduced exposure to predators and competition from other bacterial species, the ample supply of nutrients and relative environmental stability inside the host.
Genome sequencing reveals that obligate bacterial endosymbionts of insects have among the smallest of known bacterial genomes and have lost many genes that are commonly found in closely related bacteria. Several theories have been put forth to explain the loss of genes. It is presumed that some of these genes are not needed in the environment of the host insect cell. A complementary theory suggests that the relatively small numbers of bacteria inside each insect decrease the efficiency of natural selection in 'purging' deleterious mutations and small mutations from the population, resulting in a loss of genes over many millions of years. Research in which a parallel phylogeny of bacteria and insects was inferred supports the belief that the primary endosymbionts are transferred only vertically (i.e., from the mother), and not horizontally (i.e., by escaping the host and entering a new host).[13][14]
Attacking obligate bacterial endosymbionts may present a way to control their insect hosts, many of which are pests or carriers of human disease. For example, aphids are crop pests and the tsetse fly carries the organism Trypanosoma brucei that causes African sleeping sickness.[15] Other motivations for their study involve understanding the origins of symbioses in general, as a proxy for understanding e.g. how chloroplasts or mitochondria came to be obligate symbionts of eukaryotes or plants.
Secondary endosymbionts
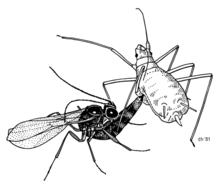
The pea aphid (Acyrthosiphon pisum) is known to contain at least three secondary endosymbionts, Hamiltonella defensa, Regiella insecticola, and Serratia symbiotica. Hamiltonella defensa defends its aphid host from parasitoid wasps.[16] This defensive symbiosis improves the survival of aphids, which have lost some elements of the insect immune response.[17]
One of the best-understood defensive symbionts is the spiral bacteria Spiroplasma poulsonii. Spiroplasma sp. can be reproductive manipulators, but also defensive symbionts of Drosophila flies. In Drosophila neotestacea, S. poulsonii has spread across North America owing to its ability to defend its fly host against nematode parasites.[18] This defence is mediated by toxins called "ribosome-inactivating proteins" that attack the molecular machinery of invading parasites.[19][20] These Spiroplasma toxins represent one of the first examples of a defensive symbiosis with a mechanistic understanding for defensive symbiosis between an insect endosymbiont and its host.
Sodalis glossinidius is a secondary endosymbiont of tsetse flies that lives inter- and intracellularly in various host tissues, including the midgut and hemolymph. Phylogenetic studies have not indicated a correlation between evolution of Sodalis and tsetse.[21] Unlike tsetse's primary symbiont Wigglesworthia, though, Sodalis has been cultured in vitro.[22]
Many other insects have secondary endosymbionts not reviewed here.[23][8]
Endosymbionts of marine invertebrates
Extracellular endosymbionts are also represented in all four extant classes of Echinodermata (Crinoidea, Ophiuroidea, Echinoidea, and Holothuroidea). Little is known of the nature of the association (mode of infection, transmission, metabolic requirements, etc.) but phylogenetic analysis indicates that these symbionts belong to the alpha group of the class Proteobacteria, relating them to Rhizobium and Thiobacillus. Other studies indicate that these subcuticular bacteria may be both abundant within their hosts and widely distributed among the Echinoderms in general.[24]
Some marine oligochaeta (e.g., Olavius algarvensis and Inanidrillus spp.) have obligate extracellular endosymbionts that fill the entire body of their host. These marine worms are nutritionally dependent on their symbiotic chemoautotrophic bacteria lacking any digestive or excretory system (no gut, mouth, or nephridia).[25]
The sea slug Elysia chlorotica lives in endosymbiotic relationship with the algae Vaucheria litorea, and the jellyfish Mastigias have a similar relationship with an algae.
Dinoflagellate endosymbionts
Dinoflagellate endosymbionts of the genus Symbiodinium, commonly known as zooxanthellae, are found in corals, mollusks (esp. giant clams, the Tridacna), sponges, and foraminifera. These endosymbionts drive the formation of coral reefs by capturing sunlight and providing their hosts with energy for carbonate deposition.[26]
Previously thought to be a single species, molecular phylogenetic evidence over the past couple decades has shown there to be great diversity in Symbiodinium. In some cases, there is specificity between host and Symbiodinium clade. More often, however, there is an ecological distribution of Symbiodinium, the symbionts switching between hosts with apparent ease. When reefs become environmentally stressed, this distribution of symbionts is related to the observed pattern of coral bleaching and recovery. Thus, the distribution of Symbiodinium on coral reefs and its role in coral bleaching presents one of the most complex and interesting current problems in reef ecology.[26]
Endosymbionts of phytoplankton
In marine environments, bacterial endosymbionts have more recently been discovered.[27][28][29][30] These endosymbiotic relationships are especially prevalent in oligotrophic or nutrient-poor regions of the ocean like that of the North Atlantic.[27][31][28][29] In these oligotrophic waters, cell growth of larger phytoplankton like that of diatoms is limited by low nitrate concentrations.[32] Endosymbiotic bacteria fix nitrogen for their diatom hosts and in turn receive organic carbon from photosynthesis.[31] These symbioses play an important role in global carbon cycling in oligotrophic regions.[33][28][29]
One known symbiosis between the diatom Hemialus spp. and the cyanobacterium Richelia intracellularis has been found in the North Atlantic, Mediterranean, and Pacific Ocean.[27][28][34] The Richelia endosymbiont is found within the diatom frustule of Hemiaulus spp., and has a reduced genome likely losing genes related to pathways the host now provides.[35] Research by Foster et al. (2011) measured nitrogen fixation by the cyanobacterial host Richelia intracellularis well above intracellular requirements, and found the cyanobacterium was likely fixing excess nitrogen for Hemiaulus host cells.[32] Additionally, both host and symbiont cell growth were much greater than free-living Richelia intracellularis or symbiont-free Hemiaulus spp.[32] The Hemaiulus-Richelia symbiosis is not obligatory especially in areas with excess nitrogen (nitrogen replete).[27]
Richelia intracellularis is also found in Rhizosolenia spp., a diatom found in oligotrophic oceans.[31][32][29] Compared to the Hemaiulus host, the endosymbiosis with Rhizosolenia is much more consistent, and Richelia intracellularis is generally found in Rhizosolenia.[27] There are some asymbiotic (occurs without an endosymbiont) Rhizosolenia, however there appears to be mechanisms limiting growth of these organisms in low nutrient conditions.[36] Cell division for both the diatom host and cyanobacterial symbiont can be uncoupled and mechanisms for passing bacterial symbionts to daughter cells during cell division are still relatively unknown.[36]
Other endosymbiosis with nitrogen fixers in open oceans include Calothrix in Chaetocerous spp. and UNCY-A in prymnesiophyte microalga.[37] The Chaetocerous-Calothrix endosymbiosis is hypothesized to be more recent, as the Calothrix genome is generally intact. While other species like that of the UNCY-A symbiont and Richelia have reduced genomes.[35] This reduction in genome size occurs within nitrogen metabolism pathways indicating endosymbiont species are generating nitrogen for their hosts and losing the ability to use this nitrogen independently.[35] This endosymbiont reduction in genome size, might be a step that occurred in the evolution of organelles (above).[37]
Endosymbionts of protists
Mixotricha paradoxa is a protozoan that lacks mitochondria. However, spherical bacteria live inside the cell and serve the function of the mitochondria. Mixotricha also has three other species of symbionts that live on the surface of the cell.
Paramecium bursaria, a species of ciliate, has a mutualistic symbiotic relationship with green alga called Zoochlorella. The algae live inside the cell, in the cytoplasm.
Paulinella chromatophora is a freshwater amoeboid which has recently (evolutionarily speaking) taken on a cyanobacterium as an endosymbiont.
Many foraminifera are hosts to several types of algae, such as red algae, diatoms, dinoflagellates and chlorophyta.[38] These endosymbionts can be transmitted vertically to the next generation via asexual reproduction of the host, but because the endosymbionts are larger than the foraminiferal gametes, they need to acquire new algae again after sexual reproduction.[39]
Several species of radiolaria have photosynthetic symbionts. In some species the host will sometimes digest algae to keep their population at a constant level.[40]
Hatena arenicola is a flagellate protist with a complicated feeding apparaturs that feed on other microbes. But when it engulf a green alga from the genus Nephroselmis, the feeding apparatus disappears and it becomes photosyntethic. During mitosis the algae is transferred to only one of the two cells, and the cell without the algae needs to start the cycle all over again.
In 1966, biologist Kwang W. Jeon found that a lab strain of Amoeba proteus had been infected by bacteria that lived inside the cytoplasmic vacuoles. This infection killed all the protists except from a few individuals. After the equivalent of 40 host generations, the two organisms gradually became mutually interdependent. Over many years of study, it has been confirmed that a genetic exchenge between the prokaryotes and protists has occurred.[41][42]
Endosymbionts of vertebrates
The spotted salamander (Ambystoma maculatum) lives in a relationship with the algae Oophila amblystomatis, which grows in the egg cases.[43]
Endosymbionts of plants
Chloroplasts are primary endosymbionts of plants that provide energy to the plant by generating sugars.
Of all the plants, Azolla has the most intimate relationship with a symbiont, as its cyanobacterium symbiont Anabaena is passed on directly from one generation to the next.[44][45]
Virus-host associations
The human genome project found several thousand endogenous retroviruses, endogenous viral elements in the genome that closely resemble and can be derived from retroviruses, organized into 24 families.[46][47]
See also
- Anagenesis
- Endophyte
- Ectosymbiosis
- List of symbiotic organisms
- List of symbiotic relationships
- Multigenomic organism
- Protocell
References
- Margulis L, Chapman MJ (2009). Kingdoms & domains an illustrated guide to the phyla of life on Earth (4th ed.). Amsterdam: Academic Press/Elsevier. p. 493. ISBN 978-0-08-092014-6.
- Mergaert P (April 2018). "Role of antimicrobial peptides in controlling symbiotic bacterial populations". Natural Product Reports. 35 (4): 336–356. doi:10.1039/c7np00056a. PMID 29393944.
- Little AF, van Oppen MJ, Willis BL (June 2004). "Flexibility in algal endosymbioses shapes growth in reef corals". Science. New York, N.Y. 304 (5676): 1492–4. Bibcode:2004Sci...304.1491L. doi:10.1126/science.1095733. PMID 15178799.
- Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H (September 2000). "Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS". Nature. 407 (6800): 81–6. Bibcode:2000Natur.407...81S. doi:10.1038/35024074. PMID 10993077.
- Bright M, Bulgheresi S (March 2010). "A complex journey: transmission of microbial symbionts". Nature Reviews. Microbiology. 8 (3): 218–30. doi:10.1038/nrmicro2262. PMC 2967712. PMID 20157340.
- Moore KR, Magnabosco C, Momper L, Gold DA, Bosak T, Fournier GP (2019). "An Expanded Ribosomal Phylogeny of Cyanobacteria Supports a Deep Placement of Plastids". Frontiers in Microbiology. 10: 1612. doi:10.3389/fmicb.2019.01612. PMC 6640209. PMID 31354692.
- Sagan L (March 1967). "On the origin of mitosing cells". Journal of Theoretical Biology. 14 (3): 255–74. doi:10.1016/0022-5193(67)90079-3. PMID 11541392.
- Wernegreen JJ (November 2002). "Genome evolution in bacterial endosymbionts of insects". Nature Reviews. Genetics. 3 (11): 850–61. doi:10.1038/nrg931. PMID 12415315.
- Campbell MA, Łukasik P, Simon C, McCutcheon JP (November 2017). "Idiosyncratic Genome Degradation in a Bacterial Endosymbiont of Periodical Cicadas". Current Biology. 27 (22): 3568–3575.e3. doi:10.1016/j.cub.2017.10.008. PMID 29129532.
- Baumann P, Moran NA, Baumann L (2000). "Bacteriocyte-associated endosymbionts of insects". In Dworkin M (ed.). The prokaryotes. New York: Springer.
- Douglas AE (1998). "Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera". Annual Review of Entomology. 43: 17–37. doi:10.1146/annurev.ento.43.1.17. PMID 15012383. S2CID 29594533.
- Douglas AE (January 1998). "Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera". Annual Review of Entomology. 43: 17–37. doi:10.1146/annurev.ento.43.1.17. PMID 15012383. S2CID 29594533.
- Wernegreen JJ (March 2004). "Endosymbiosis: lessons in conflict resolution". PLOS Biology. 2 (3): E68. doi:10.1371/journal.pbio.0020068. PMC 368163. PMID 15024418.
- Moran NA (April 1996). "Accelerated evolution and Muller's rachet in endosymbiotic bacteria". Proceedings of the National Academy of Sciences of the United States of America. 93 (7): 2873–8. Bibcode:1996PNAS...93.2873M. doi:10.1073/pnas.93.7.2873. PMC 39726. PMID 8610134.
- Aksoy S, Maudlin I, Dale C, Robinson AS, O'Neill SL (January 2001). "Prospects for control of African trypanosomiasis by tsetse vector manipulation". Trends in Parasitology. 17 (1): 29–35. doi:10.1016/S1471-4922(00)01850-X. PMID 11137738.
- Oliver KM, Campos J, Moran NA, Hunter MS (February 2008). "Population dynamics of defensive symbionts in aphids". Proceedings. Biological Sciences. 275 (1632): 293–9. doi:10.1098/rspb.2007.1192. PMC 2593717. PMID 18029301.
- International Aphid Genomics Consortium (February 2010). "Genome sequence of the pea aphid Acyrthosiphon pisum". PLOS Biology. 8 (2): e1000313. doi:10.1371/journal.pbio.1000313. PMC 2826372. PMID 20186266.
- Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ (July 2010). "Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont". Science. 329 (5988): 212–5. Bibcode:2010Sci...329..212J. doi:10.1126/science.1188235. PMID 20616278. S2CID 206526012.
- Hamilton PT, Peng F, Boulanger MJ, Perlman SJ (January 2016). "A ribosome-inactivating protein in a Drosophila defensive symbiont". Proceedings of the National Academy of Sciences of the United States of America. 113 (2): 350–5. Bibcode:2016PNAS..113..350H. doi:10.1073/pnas.1518648113. PMC 4720295. PMID 26712000.
- Ballinger MJ, Perlman SJ (July 2017). "Generality of toxins in defensive symbiosis: Ribosome-inactivating proteins and defense against parasitic wasps in Drosophila". PLOS Pathogens. 13 (7): e1006431. doi:10.1371/journal.ppat.1006431. PMC 5500355. PMID 28683136.
- Aksoy, S., Pourhosseini, A. & Chow, A. 1995. Mycetome endosymbionts of tsetse flies constitute a distinct lineage related to Enterobacteriaceae. Insect Mol Biol. 4, 15–22.
- Welburn SC, Maudlin I, Ellis DS (June 1987). "In vitro cultivation of rickettsia-like-organisms from Glossina spp". Annals of Tropical Medicine and Parasitology. 81 (3): 331–5. doi:10.1080/00034983.1987.11812127. PMID 3662675.
- Zchori-Fein E, Perlman SJ (July 2004). "Distribution of the bacterial symbiont Cardinium in arthropods". Molecular Ecology. 13 (7): 2009–16. doi:10.1111/j.1365-294X.2004.02203.x. PMID 15189221.
- Burnett WJ, McKenzie JD (May 1997). "Subcuticular bacteria from the brittle star Ophiactis balli (Echinodermata: Ophiuroidea) represent a new lineage of extracellular marine symbionts in the alpha subdivision of the class Proteobacteria". Applied and Environmental Microbiology. 63 (5): 1721–4. doi:10.1128/AEM.63.5.1721-1724.1997. PMC 168468. PMID 9143108.
- Dubilier N, Mülders C, Ferdelman T, de Beer D, Pernthaler A, Klein M, Wagner M, Erséus C, Thiermann F, Krieger J, Giere O, Amann R (May 2001). "Endosymbiotic sulphate-reducing and sulphide-oxidizing bacteria in an oligochaete worm". Nature. 411 (6835): 298–302. Bibcode:2001Natur.411..298D. doi:10.1038/35077067. PMID 11357130.
- Baker AC (November 2003). "FLEXIBILITY AND SPECIFICITY IN CORAL-ALGAL SYMBIOSIS: Diversity, Ecology, and Biogeography of Symbiodinium". Annual Review of Ecology, Evolution, and Systematics. 34: 661–89. doi:10.1146/annurev.ecolsys.34.011802.132417. S2CID 35278104.
- Villareal T (1994). "Widespread occurrence of the Hemiaulus-cyanobacterial symbiosis in the southwest North Atlantic Ocean". Bulletin of Marine Science. 54: 1–7.
- Carpenter EJ, Montoya JP, Burns J, Mulholland MR, Subramaniam A, Capone DG (20 August 1999). "Extensive bloom of a N2-fixing diatom/cyanobacterial association in the tropical Atlantic Ocean". Marine Ecology Progress Series. 185: 273–283. Bibcode:1999MEPS..185..273C. doi:10.3354/meps185273.
- Foster RA, Subramaniam A, Mahaffey C, Carpenter EJ, Capone DG, Zehr JP (March 2007). "Influence of the Amazon River plume on distributions of free-living and symbiotic cyanobacteria in the western tropical north Atlantic Ocean". Limnology and Oceanography. 52 (2): 517–532. Bibcode:2007LimOc..52..517F. doi:10.4319/lo.2007.52.2.0517. S2CID 53504106.
- Subramaniam A, Yager PL, Carpenter EJ, Mahaffey C, Björkman K, Cooley S, Kustka AB, Montoya JP, Sañudo-Wilhelmy SA, Shipe R, Capone DG (July 2008). "Amazon River enhances diazotrophy and carbon sequestration in the tropical North Atlantic Ocean". Proceedings of the National Academy of Sciences of the United States of America. 105 (30): 10460–5. doi:10.1073/pnas.0710279105. PMC 2480616. PMID 18647838.
- Goebel NL, Turk KA, Achilles KM, Paerl R, Hewson I, Morrison AE, Montoya JP, Edwards CA, Zehr JP (December 2010). "Abundance and distribution of major groups of diazotrophic cyanobacteria and their potential contribution to N₂ fixation in the tropical Atlantic Ocean". Environmental Microbiology. 12 (12): 3272–89. doi:10.1111/j.1462-2920.2010.02303.x. PMID 20678117.
- Foster RA, Kuypers MM, Vagner T, Paerl RW, Musat N, Zehr JP (September 2011). "Nitrogen fixation and transfer in open ocean diatom-cyanobacterial symbioses". The ISME Journal. 5 (9): 1484–93. doi:10.1038/ismej.2011.26. PMC 3160684. PMID 21451586.
- Scharek R, Tupas LM, Karl DM (11 June 1999). "Diatom fluxes to the deep sea in the oligotrophic North Pacific gyre at Station ALOHA". Marine Ecology Progress Series. 182: 55–67. Bibcode:1999MEPS..182...55S. doi:10.3354/meps182055.
- Zeev EB, Yogev T, Man-Aharonovich D, Kress N, Herut B, Béjà O, Berman-Frank I (September 2008). "Seasonal dynamics of the endosymbiotic, nitrogen-fixing cyanobacterium Richelia intracellularis in the eastern Mediterranean Sea". The ISME Journal. 2 (9): 911–23. doi:10.1038/ismej.2008.56. PMID 18580972.
- Hilton JA, Foster RA, Tripp HJ, Carter BJ, Zehr JP, Villareal TA (23 April 2013). "Genomic deletions disrupt nitrogen metabolism pathways of a cyanobacterial diatom symbiont". Nature Communications. 4 (1): 1767. Bibcode:2013NatCo...4.1767H. doi:10.1038/ncomms2748. PMC 3667715. PMID 23612308.
- Villareal TA (December 1989). "Division cycles in the nitrogen-fixingRhizosolenia(Bacillariophyceae)-Richelia(Nostocaceae) symbiosis". British Phycological Journal. 24 (4): 357–365. doi:10.1080/00071618900650371.
- Zehr JP (September 2015). "EVOLUTION. How single cells work together". Science. 349 (6253): 1163–4. doi:10.1126/science.aac9752. PMID 26359387.
- Joseph Seckbach; Patrick Kociolek (2011). The Diatom World. Springer Science & Business Media. p. 439. ISBN 978-94-007-1327-7.
- Origins and early evolution of photosynthetic eukaryotes – Semantic Scholar
- Surindar Paracer; Vernon Ahmadjian (2000). Symbiosis: An Introduction to Biological Associations. Oxford University Press. p. 155. ISBN 978-0-19-511807-0.
- Kwang W. Jeon | Biochemistry & Cellular and Molecular Biology – UTK BCMB
- Luigi Nibali; Brian Henderson (2016). The Human Microbiota and Chronic Disease: Dysbiosis as a Cause of Human Pathology. John Wiley & Sons. p. 165. ISBN 978-1-118-98287-7.
- Kerney R, Kim E, Hangarter RP, Heiss AA, Bishop CD, Hall BK (April 2011). "Intracellular invasion of green algae in a salamander host". Proceedings of the National Academy of Sciences of the United States of America. 108 (16): 6497–502. Bibcode:2011PNAS..108.6497K. doi:10.1073/pnas.1018259108. PMC 3080989. PMID 21464324.
- Li FW, Brouwer P, Carretero-Paulet L, Cheng S, de Vries J, Delaux PM, et al. (July 2018). "Fern genomes elucidate land plant evolution and cyanobacterial symbioses". Nature Plants. 4 (7): 460–472. doi:10.1038/s41477-018-0188-8. PMC 6786969. PMID 29967517.
- "Why is Azolla unique? | The Azolla Foundation". theazollafoundation.org. Retrieved 10 February 2020.
- Villarreal LP (October 2001). "Persisting Viruses Could Play Role in Driving Host Evolution". ASM News. Archived from the original on 8 May 2009.
- Belshaw R, Pereira V, Katzourakis A, Talbot G, Paces J, Burt A, Tristem M (April 2004). "Long-term reinfection of the human genome by endogenous retroviruses". Proceedings of the National Academy of Sciences of the United States of America. 101 (14): 4894–9. Bibcode:2004PNAS..101.4894B. doi:10.1073/pnas.0307800101. PMC 387345. PMID 15044706.