Electrochromism
Electrochromism is the phenomenon where the color or opacity of a material changes when a voltage is applied. By doing so, an electrochromic smart window can block ultraviolet, visible or (near) infrared light instantaneously and on demand. The ability to control transmittance of near infrared light can increase the energy efficiency of a building, reducing the amount of energy needed to cool during summer and heat during winter.[1]
As the color change is persistent and energy need only be applied to effect a change, electrochromic materials are used to control the amount of light and heat allowed to pass through a surface, most commonly smart windows. One popular application is in the automobile industry where it is used to automatically tint rear-view mirrors in various lighting conditions.
Principle
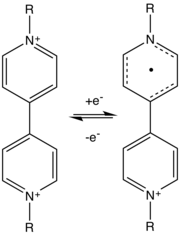
The phenomenon of electrochromism occurs in some transition metal oxides which conduct both electricity and ions, such as tungsten trioxide (WO3).[2] These oxides have octahedral structures of oxygen which surround a central metal atom and are joined together at the corners. This arrangement results in a three-dimensional nanoporous structure with "tunnels" between individual octahedral segments.[3] These tunnels allow dissociated ions to pass through the substance when they are motivated by an electric field. Common ions used for this purpose are H+ and Li+.
The electric field is typically induced by two flat, transparent electrodes which sandwich the ion-containing layers. As a voltage is applied across these electrodes, the difference in charge between the two sides causes the ions to penetrate the oxide as the charge-balancing electrons flow between the electrodes. These electrons change the valency of the metal atoms in the oxide, reducing their charge, as in the following example of tungsten trioxide:[4]
- WO
3 + n(H+
+ e−) → H
nWO
3
This is a redox reaction, since the electroactive metal is accepting electrons from the electrodes, forming a half-cell.[4] Strictly speaking the electrode as a chemical unit comprises the flat plate as well as the semiconducting substance in contact with it. However, the term electrode often refers to only the flat plate(s), more specifically called the electrode substrate.
Photons which reach the oxide layer can cause an electron to move between two nearby metal ions. The energy provided by the photon causes movement of an electron which in turn causes optical absorption of the photon. For example, the following process occurs in tungsten oxide for two tungsten ions a and b:
- W5+
a + W6+
b + photon → W6+
a + W5+
b
Electrochromic materials
Electrochromic materials, also known as chromophores, affect the optical color or opacity of a surface when a voltage is applied.[4] Among the metal oxides, tungsten oxide (WO3) is the most extensively studied and well-known electrochromic material. Others include molybdenum, titanium and niobium oxides, although these are less effective optically.
For organic materials, viologens have been commercialized on small scale. A variety of conducting polymers are also of interest, including polypyrrole, PEDOT, and polyaniline. Viologen is used in conjunction with titanium dioxide (TiO2, also known as titania) in the creation of small digital displays. It is hoped that these displays will replace liquid crystal displays as the viologen, which is typically dark blue, provides a higher contrast than the bright white of titanium dioxide, thereby increasing the visibility of a display.
Synthesis of tungsten oxide
Many methods have been used to synthesize tungsten oxide, including chemical vapor deposition (CVD), sputtering, thermal evaporation, spray pyrolysis (from a vapor or sol-gel), and hydrothermal synthesis (from a liquid).[5] In industry, sputtering is the most common method for the deposition of tungsten oxide. For material synthesis, sol-gel process is widely used due to its advantages of simple process, low cost and easy control.[6]
Sol-gel process
In the sol-gel process of tungsten trioxide, WCl
6 is dissolved in alcohol and then oxidized by purging O
2 into its solution:
- 2WCl
6 + 3O
2 → 3WO
3 + 6Cl
2
The formation of H
2 is performed by the reaction of alcohol and chlorine that used for the reduction of WO
3 to obtain a blue solution of HWO
3:
- (CH
3)
2CH–OH + 3Cl
2 → (Cl
3C)
2=O + 4H
2
- 2WO
3 + H
2 → 2HWO
3
WO
3 nanoparticles can also be obtained by precipitation of ammonium tungstate para pentahydrate, (NH
4)
10W
12O
41⋅5H
2O, or nitric acid, HNO
3, under acidic conditions from aqueous solutions.[7]
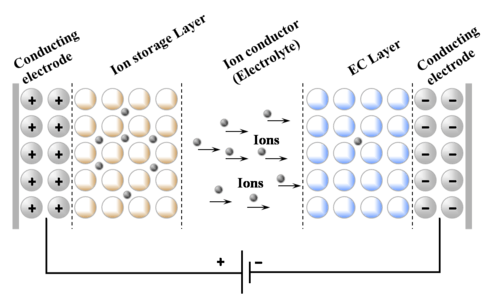
Working principle of electrochromic windows
Seven layers are needed for a functional smart window with electrochromic characteristics. The first and last are transparent glass made of silica (SiO
2), the two electrodes are needed to apply the voltage, which in turn will push (or pull) Li+
ions from the ion storage layer, through the electrolyte into the electrochromic material (or vice versa). Applying a high voltage (4 V or more) will push lithium-ions into the electrochromic layer, deactivating the electrochromic material. The window is fully transparent now. By applying a lower voltage (2.5 V for example) the concentration of Li-ions in the electrochromic layer decreases, thus activating (N)IR-active tungsten oxide. This activation causes reflection of infrared light, thus lowering the greenhouse effect, which in turn reduces the amount of energy needed for air conditioning. Depending on the electrochromic material used, different parts of the spectrum can be blocked, this way UV, VIS and IR can be independently reflected at the will of a customer.
Applications
.jpg)
Several electrochromic devices have been developed. Electrochromism is commonly used in the production of electrochromic windows or "smart glass", and more recently electrochromic displays on paper substrate as anti-counterfeiting systems integrated into packaging. NiO materials have been widely studied as counter electrodes for complementary electrochromic devices, particularly for smart windows.
ICE 3 high speed trains use electrochromic glass panels between the passenger compartment and the driver's cabin. The standard mode is clear, and can be switched by the driver to frosted (translucent), mainly to conceal unsightly collisions from the view of passengers. Electrochromic windows are used in the Boeing 787 Dreamliner.
Further reading
- Granqvist, C.G. (2002) [1995]. Handbook of Inorganic Electrochromic Materials. Elsevier. ISBN 978-0-08-053290-5.
- Lin, Feng; Nordlund, Dennis; Weng, Tsu-Chien; et al. (2013). "Origin of Electrochromism in High-Performing Nanocomposite Nickel Oxide". ACS Applied Materials & Interfaces. American Chemical Society. 5 (9): 3643–3649. doi:10.1021/am400105y. PMID 23547738.
- Moulki, Hakim; Park, Dae Hoon; Min, Bong-Ki; et al. (15 July 2012). "Improved electrochromic performances of NiO based thin films by lithium addition: From single layers to devices". Electrochimica Acta. 74: 46–52. doi:10.1016/j.electacta.2012.03.123.
- Lin, Feng; Cheng, Jifang; Engtrakul, Chaiwat; et al. (2012). "In situ crystallization of high performing WO3-based electrochromic materials and the importance for durability and switching kinetics". Journal of Materials Chemistry. 22 (33): 16817–16823. doi:10.1039/c2jm32742b.
- Deb, S. K. (1969). "A Novel Electrophotographic System". Applied Optics. 8 (S1): 192–195. Bibcode:1969ApOpt...8S.192D. doi:10.1364/AO.8.S1.000192.
- Deb, S. K. (1973). "Optical and photoelectric properties and colour centres in thin films of tungsten oxide". Philosophical Magazine. 27 (4): 801–822. Bibcode:1973PMag...27..801D. doi:10.1080/14786437308227562.
- Gillaspie, Dane T.; Tenent, Robert C.; Dillon, Anne C. (2010). "Metal-oxide films for electrochromic applications: present technology and future directions". Journal of Materials Chemistry. 20 (43): 9585–9592. doi:10.1039/C0JM00604A.
- Danine, A.; Cojocaru, L.; Faure, C.; et al. (20 May 2014). "Room Temperature UV treated WO3 thin films for electrochromic devices on paper substrate". Electrochimica Acta. 129: 113–119. doi:10.1016/j.electacta.2014.02.028.
- WO patent 2014135804, Danine, Abdelaadim; Faure, Cyril & Campet, Guy et al., "Electrochromic device comprising three or four layers", issued September 12, 2014
References
- Mortimer, R.J. (2011). "Electrochromic Materials". Annu. Rev. Mater. Res. 41. pp. 241–268. Bibcode:2011AnRMS..41..241M. doi:10.1146/annurev-matsci-062910-100344.
- Somani, Prakash R.; Radhakrishnan, S. (26 September 2001). "Electrochromic materials and devices: present and future" (PDF). Materials Chemistry and Physics. Elsevier. 77: 117–133. doi:10.1016/S0254-0584(01)00575-2. Retrieved 22 August 2019.
- Granqvist, C.G. (2015). "Fenestration for reducing building cooling needs". Eco-efficient Materials for Mitigating Building Cooling Needs. Elsevier. pp. 460–464. ISBN 978-1-78242-380-5.
- Monk, P.M.S.; Mortimer, R.J.; Rosseinsky, D.R. (2007). Electrochromism and Electrochromic Devices. Cambridge University Press. ISBN 978-0-521-82269-5.
- Zheng, Haidong; Ou, Jian Zhen; Strano, Michael S.; Kaner, Richard B.; Mitchell, Arnan; Kalantar-zadeh, Kourosh (2011-05-24). "Nanostructured Tungsten Oxide – Properties, Synthesis, and Applications". Advanced Functional Materials. 21 (12): 2175–2196. doi:10.1002/adfm.201002477. ISSN 1616-301X.
- Lai, Wei Hao; Su, Yen Hsun; Teoh, Lay Gaik; Tsai, Yuan Tsung; Hon, Min Hsiung (2007). "Synthesis of Tungsten Oxide Particles by Chemical Deposition Method". Materials Transactions. 48 (6): 1575–1577. doi:10.2320/matertrans.mep2007057. ISSN 1345-9678.
- Supothina, Sitthisuntorn; Seeharaj, Panpailin; Yoriya, Sorachon; Sriyudthsak, Mana (August 2007). "Synthesis of tungsten oxide nanoparticles by acid precipitation method". Ceramics International. 33 (6): 931–936. doi:10.1016/j.ceramint.2006.02.007. ISSN 0272-8842.
External links
- Tutorial on electrochromatic displays at Gent University (archived from the original on 6 January 2012)
- Article on energy efficiency of electrochromic windows at National Renewable Energy Laboratory (archived from the original on 21 July 2017)
- Video of electrochromic glass changing from translucent to transparent at YouTube