Viologen
Viologens are organic compounds with the formula (C5H4NR)2n+. In some viologens, the pyridyl groups are further modified.[1]
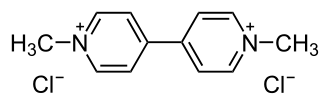
The viologen paraquat (R = methyl), is a widely used herbicide.
Other viologens have been commercialized because they can change color reversibly many times through reduction and oxidation. The name viologen alludes to violet, one color it can exhibit, and the radical cation (C5H4NR)2+ is colored intensely blue.
Types of viologens
As bipyridinium derivatives, the viologens are related to 4,4'-bipyridyl. The basic nitrogen centers in these compounds are alkylated to give viologens:
- (C5H4N)2 + 2 RX → [(C5H4NR)2]2+(X−)2
The alkylation is a form of quaternization. When the alkylating agent is a small alkyl halide, such as methyl chloride or methyl bromide, the viologen salt is often water-soluble. A wide variety of alkyl substituents have been investigated. Common derivatives are methyl (see paraquat), long chain alkyl, and benzyl.
Redox properties
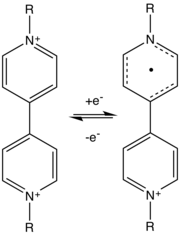
Viologens, in their dicationic form, typically undergo two one-electron reductions. The first reduction affords the deeply colored radical cation:[2]
- [V]2+ + e− [V]+
The radical cations are blue for 4,4'-viologens and green for 2,2'-derivatives. The second reduction yields a yellow quinoid compounds:
- [V]+ + e− [V]0
The electron transfer is fast because the redox process induces little structural change.
Research
Viologens have highly reversible redox reactions, and are relatively inexpensive among redox-active organic compounds. They are convenient colorimetric reagents for biochemical redox reactions.
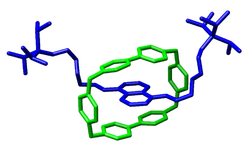
Their tendency to form host–guest complexes is key to the molecular machines recognized by the 2016 Nobel Prize in Chemistry.
Viologens are used in the negative electrolytes of some experimental flow batteries. Viologens have been modified to optimize their performance in such batteries, e.g. by incorporating them into redox-active polymers.[5]
Viologen catalysts have been reported to have the potential to oxidize glucose and other carbohydrates catalytically in a mildly alkaline solution, which makes direct carbohydrate fuel cells possible.[6]
Modified viologens and related compounds
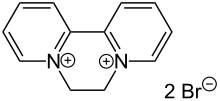
Diquat is an isomer of viologens, being derived from 2,2'-bipyridine (instead of the 4,4'-isomer). It also is a potent herbicide that functions by disrupting electron-transfer.
Extended viologens have been developed based on conjugated oligomers such as based on aryl, ethylene, and thiophene units are inserted between the pyridine units.[7] The bipolaron di-octyl bis(4-pyridyl)biphenyl viologen 2 in scheme 2 can be reduced by sodium amalgam in DMF to the neutral viologen 3.
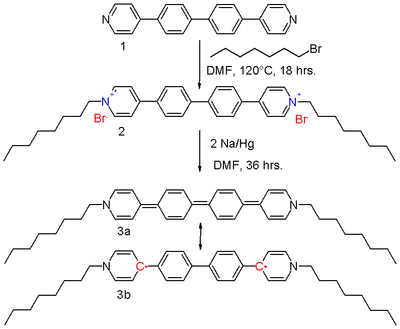
The resonance structures of the quinoid 3a and the biradical 3b contribute equally to the hybrid structure. The driving force for the contributing 3b is the restoration of aromaticity with the biphenyl unit. It has been established using X-ray crystallography that the molecule is, in effect, coplanar with slight nitrogen pyramidalization, and that the central carbon bonds are longer (144 pm) than what would be expected for a double bond (136 pm). Further research shows that the diradical exists as a mixture of triplets and singlets, although an ESR signal is absent. In this sense, the molecule resembles Tschischibabin's hydrocarbon, discovered during 1907. It also shares with this molecule a blue color in solution, and a metallic-green color as crystals.
Compound 3 is a very strong reducing agent, with a redox potential of −1.48 V.
Mechanism of action
Viologens with 2,2'-, 4,4'-, or 2,4'-bipyridylium are highly toxic because these bipyridyl molecules readily form stable free radicals.[8] The delocalization of charge, which allows for the molecule to stay as a free radical and these structures can be easily stabilized because the nitrogens can be readily hydrogenated. When in the body, these viologens interfere with electron transport chain, often causing cell death.[8][9] These molecules act as redox cycling agents and are able to transfer their electron to molecular oxygen.[10][9] Once the electron has been transferred to the molecular oxygen, it forms a superoxide radical that causes disproportionation, simultaneous reduction and oxidation.
These reactive free radicals can cause oxidative stress, which leads to cell death and one example of this is lipid peroxidation. When in a cellular system, the superoxide radicals react with unsaturated lipids, which contain a reactive hydrogen, and produce lipid hydroperoxides.[10] These lipid hydroperoxides then decompose into lipid free radicals, and causes a chain reaction of lipid peroxidation, damaging the cellular macromolecules and eventually causing cell death. The superoxide radicals have also been found to deplete NADPH, alter other redox reactions that naturally occur in the organism, and interfere with how iron is stored and released in the body.[9]
Applications
The widely used herbicide paraquat is a viologen. This application is the largest consumer of this class of compounds.
Viologens have been commercialized as electrochromic systems because of their ability to change color reversibly many times upon reduction and oxidation. In some applications, N-heptyl viologens are used. Conducting solid supports such as titania and indium tin oxide have been used.[3]
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "viologens". doi:10.1351/goldbook.V06624
- Bockman T. M.; Kochi J. K. (1990). "Isolation and oxidation-reduction of methylviologen cation radicals. Novel disproportionation in charge-transfer salts by X-ray crystallography". J. Org. Chem. 55 (13): 4127–4135. doi:10.1021/jo00300a033.
- Mortimer, R. J. (2011). "Electrochromic Materials". Annu. Rev. Mater. Res. 41. pp. 241–268. Bibcode:2011AnRMS..41..241M. doi:10.1146/annurev-matsci-062910-100344.
- Bravo, José A.; Raymo, Françisco M.; Stoddart, J. Fraser; White, Andrew J. P.; Williams, David J. (1998). "High Yielding Template-Directed Syntheses of [2]Rotaxanes". Eur. J. Org. Chem. 1998 (11): 2565–2571. doi:10.1002/(SICI)1099-0690(199811)1998:11<2565::AID-EJOC2565>3.0.CO;2-8.
- Burgess, Mark; Moore, Jeffrey S.; Rodriguez-Lopez, Joaquin (2016), "Redox Active Polymers as Soluble Nanomaterials for Energy Storage", Accounts of Chemical Research, 49 (11): 2649–2657, doi:10.1021/acs.accounts.6b00341, PMID 27673336CS1 maint: uses authors parameter (link)
- Dean R. Wheeler; Joseph Nichols; Dane Hansen; Merritt Andrus; Sang Choi & Gerald D. Watt (2009). "Viologen Catalysts for a Direct Carbohydrate Fuel Cell". J. Electrochem. Soc. 156 (10): B1201–B1207. doi:10.1149/1.3183815.
- W. W. Porter, T. P. Vaid and A. L. Rheingold (2005). "Synthesis and Characterization of a Highly Reducing Neutral "Extended Viologen" and the Isostructural Hydrocarbon 4,4' '-Di-n-octyl-p-quaterphenyl". J. Am. Chem. Soc. 127 (47): 16559–16566. doi:10.1021/ja053084q. PMID 16305245.
- Moreland, D. E. (1 January 1980). "Mechanisms of Action of Herbicides". Annual Review of Plant Physiology. 31 (1): 597–638. doi:10.1146/annurev.pp.31.060180.003121.
- Roede, J. R.; Miller, G. W. (1 January 2014). Diquat. Encyclopedia of Toxicology (Third Edition). pp. 202–204. doi:10.1016/B978-0-12-386454-3.00137-8. ISBN 9780123864550.
- Bus, J S; Aust, S D; Gibson, J E (1 August 1976). "Paraquat toxicity: proposed mechanism of action involving lipid peroxidation". Environmental Health Perspectives. 16: 139–146. doi:10.1289/ehp.7616139. ISSN 0091-6765. PMC 1475222. PMID 1017417.