Centanafadine
Centanafadine (INN) (former developmental code name EB-1020) is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) that began its development with Euthymics Bioscience after they acquired DOV Pharmaceutical. It was developed as a treatment for attention-deficit hyperactivity disorder (ADHD) and inhibits the reuptake of norepinephrine, dopamine, and serotonin with a ratio of 1:6:14, respectively.[1][2][3][4] In 2011, Euthymics Bioscience spun off its development of centanafadine to a new company called Neurovance.[5][6] In March 2017, Otsuka Pharmaceutical acquired Neurovance and the rights to centanafadine.[7] As of January 2018, Otsuka's pipeline indicates it is in Phase III clinical trials.[8]
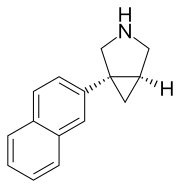 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C15H15N |
| Molar mass | 209.292 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| Site | IC50 (nM) | Action | Ref |
|---|---|---|---|
| SERT | 83 nM | Blocker | [1] |
| NET | 6 nM | Blocker | [1] |
| DAT | 38 nM | Blocker | [1] |
References
- "Neurovance's EB-1020 SR for Adult ADHD Shows Stimulant-Like Efficacy and Good Tolerability in Phase 2a Trial" (PDF). Neurovance. Retrieved 14 January 2018.
- "3-Neurotransmitters, 1-Molecule: Optimized Ratios". Neurovance.
- "EB-1020, a Non-Stimulant Norepinephrine and Dopamine - Preferring Reuptake Inhibitor for the Treatment of Adult ADHD" (PDF). Neurovance.
- Bymaster FP, Golembiowska K, Kowalska M, Choi YK, Tarazi FI (June 2012). "Pharmacological characterization of the norepinephrine and dopamine reuptake inhibitor EB-1020: implications for treatment of attention-deficit hyperactivity disorder". Synapse. 66 (6): 522–32. doi:10.1002/syn.21538. PMID 22298359.
- "Euthymics". Ethismos Research Inc. Retrieved 14 January 2018.
- "EUTHYMICS BIOSCIENCE, INC. PRESENTS DATA THAT SUPPORT ADVANCING EB-1020 INTO CLINICAL TRIALS FOR ADULT ADHD" (PDF). Neurovance. December 7, 2011. Retrieved 14 January 2018.
- "Otsuka Pharmaceutical to Acquire Neurovance, Inc". Otsuka. Retrieved 14 January 2018.
- "Otsuka U.S. Research & Development Programs". Otsuka U.S. Otsuka. Retrieved 14 January 2018.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.