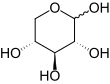
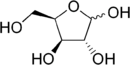
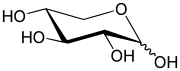
Xylose
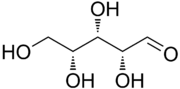
Xylose (cf. Greek: ξύλον, xylon, "wood") is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group. It is derived from hemicellulose, one of the main constituents of biomass. Like most sugars, it can adopt several structures depending on conditions. With its free aldehyde group, it is a reducing sugar.
| |||
 | |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
d-Xylose | |||
| Other names
(+)-Xylose Wood sugar | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.043.072 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
| |||
| |||
| Properties[1][2] | |||
| C5H10O5 | |||
| Molar mass | 150.13 g/mol | ||
| Appearance | monoclinic needles or prisms, colourless | ||
| Density | 1.525 g/cm3 (20 °C) | ||
| Melting point | 144 to 145 °C (291 to 293 °F; 417 to 418 K) | ||
Chiral rotation ([α]D) |
+22.5° (CHCl3) | ||
| -84.80·10−6 cm3/mol | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related aldopentoses |
Arabinose Ribose Lyxose | ||
Related compounds |
Xylulose | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Structure
The acyclic form of xylose has chemical formula HOCH2(CH(OH))3CHO. The cyclic hemiacetal isomers are more prevalent in solution and are of two types: the pyranoses, which feature six-membered C5O rings, and the furanoses, which feature five-membered C4O rings (with a pendant CH2OH group). Each of these rings is subject to further isomerism, depending on the relative orientation of the anomeric hydroxy group.
The dextrorotary form, d-xylose, is the one that usually occurs endogenously in living things. A levorotary form, l-xylose, can be synthesized.
Occurrence
Xylose is the main building block for the hemicellulose xylan, which comprises about 30% of some plants (birch for example), far less in others (spruce and pine have about 9% xylan). Xylose is otherwise pervasive, being found in the embryos of most edible plants. It was first isolated from wood by Finnish scientist, Koch, in 1881,[3] but first became commercially viable, with a price close to sucrose, in 1930.[4]
Xylose is also the first saccharide added to the serine or threonine in the proteoglycan type O-glycosylation, and, so, it is the first saccharide in biosynthetic pathways of most anionic polysaccharides such as heparan sulfate and chondroitin sulfate.[5]
Xylose is also found in some species of Chrysolinina beetles, including Chrysolina coerulans, they have cardiac glycosides (including Xlyose) in their defensive glands.[6]
Applications
Chemicals
The acid-catalysed degradation of hemicellulose gives furfural,[7] a precursor to synthetic polymers and to tetrahydrofuran.[8]
Human consumption
Xylose is metabolised by humans, although it is not a major human nutrient and is largely excreted by the kidneys.[9] Humans can obtain xylose only from their diet. An oxidoreductase pathway is present in eukaryotic microorganisms. Humans have enzymes called protein xylosyltransferases (XYLT1, XYLT2) which transfer xylose from UDP to a serine in the core protein of proteoglycans.
Xylose contains 2.4 calories per gram[10] (lower than glucose or sucrose, approx. 4 calories per gram).
Animal medicine
In animal medicine, xylose is used to test for malabsorption by administration in water to the patient after fasting. If xylose is detected in blood and/or urine within the next few hours, it has been absorbed by the intestines.[11]
High xylose intake on the order of approximately 100g/kg of animal body weight is relatively well tolerated in pigs, and in a similar manner to results from human studies, a portion of the xylose intake is passed out in urine undigested.[12]
Hydrogen production
In 2014 a low-temperature 50 °C (122 °F), atmospheric-pressure enzyme-driven process to convert xylose into hydrogen with nearly 100% of the theoretical yield was announced. The process employs 13 enzymes, including a novel polyphosphate xylulokinase (XK).[13][14]
Derivatives
Reduction of xylose by catalytic hydrogenation produces the sugar substitute xylitol.
References
- The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, ISBN 091191028X, 9995.
- Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-574. ISBN 0-8493-0462-8..
- Advances in carbohydrate chemistry, Volume 5, pg 278 Hudson & Cantor 1950
- Pentose Metabolism 1932
- Buskas, Therese; Ingale, Sampat; Boons, Geert-Jan (2006), "Glycopeptides as versatile tool for glycobiology", Glycobiology, 16 (8): 113R–36R, doi:10.1093/glycob/cwj125, PMID 16675547
- E. David Morgan Biosynthesis in Insects (2004), p. 112, at Google Books
- Roger Adams and V. Voorhees (1921). "Furfural". Organic Syntheses. 1: 49.; Collective Volume, 1, p. 280
- Hoydonckx, H. E.; Van Rhijn, W. M.; Van Rhijn, W.; De Vos, D. E.; Jacobs, P. A. (2007). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_119.pub2.
- Johnson, S. A. (2006). "Thesis" (PDF).
- , "Method of producing xylose", issued 1999-08-06
- "D-xylose absorption", MedlinePlus, U.S. National Library of Medicine, July 2008, retrieved 2009-09-06
- Nutritional implications of D-Xylose in pigs
- "Virginia Tech team develops process for high-yield production of hydrogen from xylose under mild conditions". Green Car Congress. 2013-04-03. doi:10.1002/anie.201300766. Retrieved 2014-01-22.
- Martín Del Campo, J. S.; Rollin, J.; Myung, S.; Chun, Y.; Chandrayan, S.; Patiño, R.; Adams, M. W.; Zhang, Y. -H. P. (2013). "High-Yield Production of Dihydrogen from Xylose by Using a Synthetic Enzyme Cascade in a Cell-Free System". Angewandte Chemie International Edition. 52 (17): 4587. doi:10.1002/anie.201300766. PMID 23512726.