Chameleon
Chameleons or chamaeleons (family Chamaeleonidae) are a distinctive and highly specialized clade of Old World lizards with 202 species described as of June 2015.[1] These species come in a range of colors, and many species have the ability to change color.
| Chameleons | |
|---|---|
_Photograph_By_Shantanu_Kuveskar.jpg) | |
| Indian chameleon, Chamaeleo zeylanicus, in Mangaon, Maharashtra, India | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Suborder: | Iguania |
| Clade: | Acrodonta |
| Family: | Chamaeleonidae Rafinesque, 1815 |
| Genera | |
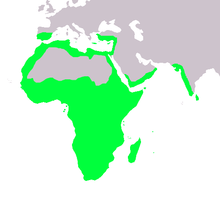 | |
| Native range of Chamaeleonidae | |
Chameleons are distinguished by their zygodactylous feet; their very extensive, highly modified, rapidly extrudable tongues; their swaying gait;[2] and crests or horns on their brow and snout. Most species, the larger ones in particular, also have a prehensile tail. Chameleons' eyes are independently mobile, but in aiming at a prey item, they focus forward in coordination, affording the animal stereoscopic vision.
Chameleons are adapted for climbing and visual hunting. They live in warm habitats that range from rain forest to desert conditions, with various species occurring in Africa, Madagascar, southern Europe, and across southern Asia as far as Sri Lanka. They also have been introduced to Hawaii, California, and Florida, and often are kept as household pets.
Etymology

The English word chameleon (/kəˈmiːliən/ kuh-MEEL-ee-un) is a simplified spelling of Latin chamaeleōn,[3] a borrowing of the Greek χαμαιλέων (khamailéōn),[4] a compound of χαμαί (khamaí) "on the ground"[5] and λέων (léōn) "lion".[6][7][8]
Classification
The family Chamaeleonidae was divided into two subfamilies, Brookesiinae and Chamaeleoninae, by Klaver and Böhme in 1986.[9] Under this classification, Brookesiinae included the genera Brookesia and Rhampholeon, as well as the genera later split off from them (Palleon and Rieppeleon), while Chamaeleoninae included the genera Bradypodion, Calumma, Chamaeleo, Furcifer and Trioceros, as well as the genera later split off from them (Archaius, Nadzikambia and Kinyongia). Since that time, however, the validity of this subfamily designation has been the subject of much debate,[10] although most phylogenetic studies support the notion that the pygmy chameleons of the subfamily Brookesiinae are not a monophyletic group.[11][12][13][14]
While some authorities have previously preferred to use this subfamilial classification on the basis of the absence of evidence principle,[10] these authorities later abandoned this subfamilial division, no longer recognizing any subfamilies with the family Chamaeleonidae.[15]
In 2015, however, Glaw reworked the subfamilial division by placing only the genera Brookesia and Palleon within the Brookesiinae subfamily, with all other genera being placed in Chamaeleoninae.[1]

Change of color
.jpg)
Some chameleon species are able to change their skin colouration. Different chameleon species are able to vary their colouration and pattern through combinations of pink, blue, red, orange, green, black, brown, light blue, yellow, turquoise, and purple.[16] Chameleon skin has a superficial layer which contains pigments, and under the layer are cells with guanine crystals. Chameleons change colour by changing the space between the guanine crystals, which changes the wavelength of light reflected off the crystals which changes the colour of the skin.
Colour change in chameleons has functions in camouflage, but most commonly in social signaling and in reactions to temperature and other conditions. The relative importance of these functions varies with the circumstances, as well as the species. Colour change signals a chameleon's physiological condition and intentions to other chameleons.[17][18] Because chameleons are ectothermic, another reason why they change color is to regulate their body temperatures, either to a darker color to absorb light and heat to raise their temperature, or to a lighter color to reflect light and heat, thereby either stabilizing or lowering their body temperature.[19] Chameleons tend to show brighter colours when displaying aggression to other chameleons,[20] and darker colours when they submit or "give up".[21] Some species, particularly those of Madagascar and some African genera in rainforest habitats, have blue fluorescence in their skull tubercles, deriving from bones and possibly serving a signaling role.[22]
Some species, such as Smith's dwarf chameleon, adjust their colours for camouflage in accordance with the vision of the specific predator species (bird or snake) by which they are being threatened.[23]
The desert-dwelling Namaqua chameleon also uses colour change as an aid to thermoregulation, becoming black in the cooler morning to absorb heat more efficiently, then a lighter grey colour to reflect light during the heat of the day. It may show both colors at the same time, neatly separated left from right by the spine.
Mechanism of color change
For a long time it was thought that chameleons change colour by dispersion of pigment-containing organelles within their skin. However, research conducted in 2014 on panther chameleons has shown that pigment movement only represents part of the mechanism.[24]
Chameleons have two superimposed layers within their skin that control their colour and thermoregulation. The top layer contains a lattice of guanine nanocrystals, and by exciting this lattice the spacing between the nanocrystals can be manipulated, which in turn affects which wavelengths of light are reflected and which are absorbed. Exciting the lattice increases the distance between the nanocrystals, and the skin reflects longer wavelengths of light. Thus, in a relaxed state the crystals reflect blue and green, but in an excited state the longer wavelengths such as yellow, orange, green, and red are reflected.[25]
The skin of a chameleon also contains some yellow pigments, which combined with the blue reflected by a relaxed crystal lattice results in the characteristic green color which is common of many chameleons in their relaxed state. Chameleon color palettes have evolved through evolution and environment. Chameleons living in forest, have a more defined and colorful palette compared to those living in the desert or savanna; which have more of a basic, brown and charred palette.[26]
Evolution
%2C_Vohimana_reserve%2C_Madagascar.jpg)
The oldest described chameleon is Anqingosaurus brevicephalus from the Middle Paleocene (about 58.7–61.7 mya) of China.[27]
Other chameleon fossils include Chamaeleo caroliquarti from the Lower Miocene (about 13–23 mya) of the Czech Republic and Germany, and Chamaeleo intermedius from the Upper Miocene (about 5–13 mya) of Kenya.[27]
The chameleons are probably far older than that, perhaps sharing a common ancestor with iguanids and agamids more than 100 mya (agamids being more closely related). Since fossils have been found in Africa, Europe and Asia, chameleons were certainly once more widespread than they are today.
Although nearly half of all chameleon species today live in Madagascar, this offers no basis for speculation that chameleons might originate from there.[28] In fact, it has recently been shown that chameleons most likely originated in mainland Africa.[14] It appears there were two distinct oceanic migrations from the mainland to Madagascar. The diverse speciation of chameleons has been theorized to have directly reflected the increase in open habitats (savannah, grassland, and heathland) that accompanied the Oligocene period. Monophyly of the family is supported by several studies.[29]
Daza et al. (2016) described a small (10,6 mm in snout-vent length), probably neonatal lizard preserved in the Cretaceous (Albian-Cenomanian boundary) amber from Myanmar. The authors noted that the lizard has "short and wide skull, large orbits, elongated and robust lingual process, frontal with parallel margins, incipient prefrontal boss, reduced vomers, absent retroarticular process, low presacral vertebral count (between 15 and 17) and extremely short, curled tail"; the authors considered these traits to be indicative of the lizard's affiliation with Chamaeleonidae. The phylogenetic analysis conducted by the authors indicated that the lizard was a stem-chamaeleonid.[30] However, Matsumoto & Evans (2018) reinterpreted this specimen as an albanerpetontid amphibian.[31]
While the exact evolutionary history of color change in chameleons is still unknown, there is one aspect of the evolutionary history of chameleon color change that has already been conclusively studied: the effects of signal efficacy. Signal efficacy, or how well the signal can be seen against its background, has been shown to correlate directly to spectral qualities of chameleon displays.[32] Dwarf chameleons, the chameleon of study, occupy a wide variety of habitats from forests to grasslands to shrubbery. It was demonstrated that chameleons in brighter areas tended to present brighter signals, but chameleons in darker areas tended to present relatively more contrasting signals to their backgrounds. This finding suggests that signal efficacy (and thus habitat) has affected the evolution of chameleon signaling. Stuart-Fox et al. note that it makes sense that selection for crypsis is not seen to be as important as selection for signal efficacy, because the signals are only shown briefly; chameleons are most always in muted, cryptic colors.[32]
Description

Chameleons vary greatly in size and body structure, with maximum total lengths varying from 15 mm (0.59 in) in male Brookesia micra (one of the world's smallest reptiles) to 68.5 cm (27.0 in) in the male Furcifer oustaleti.[33] Many have head or facial ornamentation, such as nasal protrusions, or horn-like projections in the case of Trioceros jacksonii, or large crests on top of their heads, like Chamaeleo calyptratus. Many species are sexually dimorphic, and males are typically much more ornamented than the female chameleons.
Typical sizes of species of chameleon commonly kept in captivity or as pets are:
| Scientific name | Common name | Length (male) | Length (female) | Color | Lifespan (years) |
|---|---|---|---|---|---|
| Chamaeleo calyptratus | Veiled chameleon | 35–60 cm | 25–33 cm | Green and light colors | about 5 |
| Trioceros jacksonii | Jackson's chameleon | 23–33 cm | 25–33 cm | Green and light colors | 5–10 |
| Furcifer pardalis | Panther chameleon | 38–53 cm | 23–33 cm | Darker colors | about 5 (2–3 for birthing females) |
| Rieppeleon brevicaudatus | Bearded pygmy chameleon | 5–8 cm | 5–8 cm | Brown, beige, green | about 3–5 |
| Rhampholeon spectrum | Spectral pygmy chameleon | 8–10 cm | 5–10 cm | Tan and gray | 3–5 |
| Rhampholeon temporalis | Usambara pitted pygmy chameleon | 6–10 cm | 5–9 cm | Gray and brown | 5–11 |
The feet of chameleons are highly adapted to arboreal locomotion, and species such as Chamaeleo namaquensis that have secondarily adopted a terrestrial habit have retained the same foot morphology with little modification. On each foot, the five clearly distinguished toes are grouped into two fascicles. The toes in each fascicle are bound into a flattened group of either two or three, giving each foot a tongs-like appearance. On the front feet, the outer, lateral, group contains two toes, whereas the inner, medial, group contains three. On the rear feet, this arrangement is reversed, the medial group containing two toes, and the lateral group three. These specialized feet allow chameleons to grip tightly onto narrow or rough branches. Furthermore, each toe is equipped with a sharp claw to afford a grip on surfaces such as bark when climbing. It is common to refer to the feet of chameleons as didactyl or zygodactyl, though neither term is fully satisfactory, both being used in describing totally different feet, such as the zygodactyl feet of parrots or didactyl feet of sloths or ostriches, none of which is significantly like chameleon feet. Although "zygodactyl" is reasonably descriptive of chameleon foot anatomy, their foot structure does not resemble that of parrots, to which the term was first applied. As for didactyly, chameleons visibly have five toes on each foot, not two.
Some chameleons have a crest of small spikes extending along the spine from the proximal part of the tail to the neck; both the extent and size of the spikes varies between species and individuals. These spikes help break up the definitive outline of the chameleon, which aids it when trying to blend into a background.
Senses
Chameleons have the most distinctive eyes of any reptile. The upper and lower eyelids are joined, with only a pinhole large enough for the pupil to see through. Each eye can pivot and focus independently, allowing the chameleon to observe two different objects simultaneously. This gives them a full 360-degree arc of vision around their bodies. Prey is located using monocular depth perception, not stereopsis.[34] Chameleons have very good eyesight for reptiles, letting them see small insects from a 5–10 meter distance. In fact, chameleons have the highest magnification (per size) of any vertebrate.[35]
Like snakes, chameleons do not have an outer or a middle ear, so there is neither an ear opening nor an eardrum. However, chameleons are not deaf: they can detect sound frequencies in the range of 200–600 Hz.[36]
Chameleons can see in both visible and ultraviolet light.[37] Chameleons exposed to ultraviolet light show increased social behavior and activity levels, are more inclined to bask and feed, and are also more likely to reproduce, as it has a positive effect on the pineal gland.
Feeding
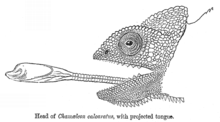



All chameleons are primarily insectivores that feed by ballistically projecting their long tongues from their mouths to capture prey located some distance away.[38] While the chameleons' tongues are typically thought to be one and a half to two times the length of their bodies (their length excluding the tail), smaller chameleons (both smaller species and smaller individuals of the same species) have recently been found to have proportionately larger tongue apparatuses than their larger counterparts.[39] Thus, smaller chameleons are able to project their tongues greater distances than the larger chameleons that are the subject of most studies and tongue length estimates, and can project their tongues more than twice their body length.[40]
The chameleon's tongue apparatus consists of highly modified hyoid bones, tongue muscles, and collagenous elements.[41][42][39][43] The hyoid bone has an elongated, parallel-sided projection, called the entoglossal process, over which a tubular muscle, the accelerator muscle, sits.[39][43][41][42] The accelerator muscle contracts around the entoglossal process and is responsible for creating the work to power tongue projection, both directly and through the loading of collagenous elements located between the entoglossal process and the accelerator muscle.[38][39][41][42] The tongue retractor muscle, the hyoglossus, connects the hyoid and accelerator muscle, and is responsible for drawing the tongue back into the mouth following tongue projection.[38][39][43][41]
Tongue projection occurs at extremely high performance, reaching the prey in as little as 0.07 seconds,[41][42][44] having been launched at accelerations exceeding 41 g.[44] The power with which the tongue is launched, known to exceed 3000 W kg−1, exceeds that which muscle is able to produce, indicating the presence of an elastic power amplifier to power tongue projection.[42] The recoil of elastic elements in the tongue apparatus is thus responsible for large percentages of the overall tongue projection performance.
One consequence of the incorporation of an elastic recoil mechanism to the tongue projection mechanism is relative thermal insensitivity of tongue projection relative to tongue retraction, which is powered by muscle contraction alone, and is heavily thermally sensitive.[44][45] While other ectothermic animals become sluggish as their body temperatures decline, due to a reduction in the contractile velocity of their muscles, chameleons are able to project their tongues at high performance even at low body temperatures.[44][45] The thermal sensitivity of tongue retraction in chameleons, however, is not a problem, as chameleons have a very effective mechanism of holding onto their prey once the tongue has come into contact with it, including surface phenomena, such as wet adhesion and interlocking, and suction.[46] The thermal insensitivity of tongue projection thus enables chameleons to feed effectively on cold mornings prior to being able to behaviorally elevate their body temperatures through thermoregulation, when other sympatric lizards species are still inactive, likely temporarily expanding their thermal niche as a result.[44]
Bones
Certain species of chameleons have bones that glow when under ultraviolet light, also known as biogenic fluorescence.[47] Some 31 different species of Calumma chameleons, all native to Madagascar, displayed this fluorescence in CT scans.[48] The bones emitted a bright blue glow and could even shine through the chameleon's four layers of skin.[48] The face was found to have a different glow, appearing as dots otherwise known as tubercles on facial bones.[47] The glow results from proteins, pigments, chitin, and other materials that make up a chameleon's skeleton,[47] possibly giving chameleons a secondary signaling system that does not interfere with their color-changing ability, and may have evolved from sexual selection.[47]
Distribution and habitat
_Lokobe.jpg)
Chameleons primarily live in the mainland of sub-Saharan Africa and on the island of Madagascar, although a few species live in northern Africa, southern Europe, the Middle East, southern India, Sri Lanka, and several smaller islands in the western Indian Ocean. There are introduced, feral populations of veiled and Jackson's chameleons in Hawaii, and isolated pockets of feral Jackson's chameleons have been reported in California, Florida and Texas.
Chameleons inhabit all kinds of tropical and mountain rain forests, savannas, and sometimes deserts and steppes. The typical chameleons from the subfamily Chamaeleoninae are arboreal, usually living in trees or bushes, although a few (notably the Namaqua chameleon) are partially or largely terrestrial. Most species from the subfamily Brookesiinae, which includes the genera Brookesia, Rieppeleon, and Rhampholeon, live low in vegetation or on the ground among leaf litter. Many species of chameleons are threatened by extinction. Declining chameleon numbers are due to habitat loss.[49]
Reproduction


Chameleons are mostly oviparous, with some being ovoviviparous.
The oviparous species lay eggs three to six weeks after copulation. The female will dig a hole — from 10–30 cm (4–12 in), deep depending on the species — and deposit her eggs. Clutch sizes vary greatly with species. Small Brookesia species may only lay two to four eggs, while large veiled chameleons (Chamaeleo calyptratus) have been known to lay clutches of 20–200 (veiled chameleons) and 10–40 (panther chameleons) eggs. Clutch sizes can also vary greatly among the same species. Eggs generally hatch after four to 12 months, again depending on species. The eggs of Parson's chameleon (Calumma parsonii), a species which is rare in captivity, are believed to take more than 24 months to hatch.[50]
The ovoviviparous species, such as the Jackson's chameleon (Trioceros jacksonii) have a five- to seven-month gestation period. Each young chameleon is born within the sticky transparent membrane of its yolk sac. The mother presses each egg onto a branch, where it sticks. The membrane bursts and the newly hatched chameleon frees itself and climbs away to hunt for itself and hide from predators. The female can have up to 30 live young from one gestation.[51]
Diet
Chameleons generally eat insects, but larger species, such as the common chameleon, may also take other lizards and young birds.[52]:5 The range of diets can be seen from the following examples:
- The veiled chameleon, Chamaeleo calyptratus from Arabia, is insectivorous, but eats leaves when other sources of water are not available. It can be maintained on a diet of crickets.[53] They can eat as many as 15–50 large crickets a day.
- Jackson's chameleon (Trioceros jacksonii) from Kenya and northern Tanzania eats a wide variety of small animals including ants, butterflies, caterpillars, snails, worms, lizards, geckos, amphibians, and other chameleons, as well as plant material, such as leaves, tender shoots, and berries. It can be maintained on a mixed diet including kale, dandelion leaves, lettuce, bananas, tomatoes, apples, crickets, and waxworms.[51]
- The common chameleon of Europe, North Africa, and the Near East, Chamaeleo chamaeleon, mainly eats wasps and mantises; such arthropods form over three quarters of its diet.[52]:5 Some experts advise that the common chameleon should not be fed exclusively on crickets; these should make up no more than half the diet, with the rest a mixture of waxworms, earthworms, grasshoppers, flies, and plant materials such as green leaves, oats, and fruit.[52]:5–6
- Temperature influences the amount of food eaten.
- Some chameleons like the panther chameleon of Madagascar regulate their vitamin D3 levels, of which their insect diet is a poor source, by exposing themselves to sunlight since its UV component increases internal production.[54]
Parasites
Chameleons are parasitized by nematode worms, including threadworms (Filarioidea). Threadworms can be transmitted by biting insects such as ticks and mosquitoes. Other roundworms are transmitted through food contaminated with roundworm eggs; the larvae burrow through the wall of the intestine into the bloodstream.[55]
Chameleons are subject to several protozoan parasites, such as Plasmodium which causes malaria, Trypanosoma which causes sleeping sickness, and Leishmania which causes leishmaniasis.[56]
Chameleons are subject to parasitism by coccidia,[56] including species of the genera Choleoeimeria, Eimeria, and Isospora.[57]
References
- Glaw, F. (2015). "Taxonomic checklist of chameleons (Squamata: Chamaeleonidae)". Vertebrate Zoology. 65 (2): 167–246.
- Edmonds, Patricia (September 2015). "True colors". National Geographic: 98.
- chamaeleon. Charlton T. Lewis and Charles Short. A Latin Dictionary on Perseus Project.
- χαμαιλέων. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- χαμαί in Liddell and Scott.
- λέων in Liddell and Scott.
- "Chameleon". Dictionary.com.
- Harper, Douglas. "chameleon". Online Etymology Dictionary.
- Klaver, C.; Böhme, W. (1986). "Phylogeny and classification of the Chamaeleonidae (Sauria) with special reference to hemipenis morphology". Bonner Zoologische Monographien. 22: 1–64.
- Tilbury, Colin (2010). Chameleons of Africa, An Atlas including the chameleons of Europe, the Middle East and Asia. Frankfurt: Edition Chimaira. ISBN 978-3899734515.
- Townsend, T.; Larson, A. (2002). "Molecular phylogenetics and mitochondrial genomic evolution in the Chamaeleonidae (Reptilia, Squamata)". Molecular Phylogenetics and Evolution. 23 (1): 22–36. doi:10.1006/mpev.2001.1076. PMID 12182400.
- Raxworthy, C. J.; Forstner, M. R. J.; Nussbaum, R. A. (2002). "Chameleon radiation by oceanic dispersal" (PDF). Nature. 415 (6873): 784–787. doi:10.1038/415784a. hdl:2027.42/62614. PMID 11845207.
- Townsend, T. M.; Tolley, K. A.; Glaw, F.; Böhme, W.; Vences, M. (2011). "Eastward from Africa: Palaeocurrent-mediated chameleon dispersal to the Seychelles islands". Biological Letters. 7 (2): 225–228. doi:10.1098/rsbl.2010.0701. PMC 3061160. PMID 20826471.
- Tolley, K. A.; Townsend, T. M.; Vences, M. (2013). "Large-scale phylogeny of chameleons suggests African origins and Eocene diversification". Proceedings of the Royal Society B. 280 (1759): 20130184. doi:10.1098/rspb.2013.0184. PMC 3619509. PMID 23536596.
- Tilbury, Colin (2014). "Overview of the Systematics of the Chamaeleonidae". In Tolley, Krystal A.; Herrel, Anthony (eds.). The Biology of Chameleons. Berkeley: University of California Press. pp. 151–174. ISBN 9780520276055.
- Chameleons. National Geographic Explorer
- Stuart-Fox, D.; Moussalli, A. (2008). "Selection for Social Signalling Drives the Evolution of Chameleon Colour Change". PLOS Biology. 6 (1): e25. doi:10.1371/journal.pbio.0060025. PMC 2214820. PMID 18232740.
- Harris, Tom. "How Animal Camouflage Works". How Stuff Works. Retrieved 2006-11-13.
- Cook, Maria. "The Adaptations of Chameleons". Sciencing. Retrieved 15 June 2020.
- Ligon, Russell A.; McGraw, Kevin J. (2013). "Chameleons communicate with complex colour changes during contests: different body regions convey different information". Biology Letters. 9 (6): 20130892. doi:10.1098/rsbl.2013.0892. PMC 3871380. PMID 24335271.
- Ligon, Russell A (2014). "Defeated chameleons darken dynamically during dyadic disputes to decrease danger from dominants". Behavioral Ecology and Sociobiology. 68 (6): 1007–1017. doi:10.1007/s00265-014-1713-z.
- David Prötzel, Martin Heß, Mark D. Scherz, Martina Schwager, Anouk van’t Padje, Frank Glaw (2018). "Widespread bone-based fluorescence in chameleons". Scientific Reports. 8 (698): 698. Bibcode:2018NatSR...8..698P. doi:10.1038/s41598-017-19070-7. PMC 5768862. PMID 29335580.CS1 maint: uses authors parameter (link)
- Young, Emma (2008) Chameleons fine-tune camouflage to predator's vision. New Scientist
- Teyssier, Jeremie; Saenko, Suzanne V.; van der Marel, Dirk; Milinkovitch, Michel C. (2015). "Photonic crystals cause active colour change in chameleons." Nature Communications (6).
- National Geographic (2015) The Colorful Language of Chameleons
- Stuart-Fox, Devi; Moussalli, Adnan (2008-01-29). "Selection for Social Signalling Drives the Evolution of Chameleon Colour Change". PLOS Biology. 6 (1): e25. doi:10.1371/journal.pbio.0060025. ISSN 1545-7885. PMC 2214820. PMID 18232740.
- Maisano, Jessie (27 August 2003). "Chamaeleo calyptratus, Veiled Chameleon". Digimorph. University of Texas at Austin. Retrieved January 10, 2012.
- Tolley, Krystal; Burger, Marius (2007). Chameleons of Southern Africa. Struik. pp. 26–28. ISBN 978-1-77007-375-3.
- Tolley, Krystal A.; Herrel, Anthony (16 November 2013). The Biology of Chameleons. Univ of California Press. ISBN 9780520276055. Retrieved 1 November 2017 – via Google Books.
- Daza, Juan D.; Stanley, Edward L.; Wagner, Philipp; Bauer, Aaron M.; Grimaldi, David A. (2016). "Mid-Cretaceous amber fossils illuminate the past diversity of tropical lizards". Science Advances. 2 (3): e1501080. Bibcode:2016SciA....2E1080D. doi:10.1126/sciadv.1501080. PMC 4783129. PMID 26973870.
- Ryoko Matsumoto; Susan E. Evans (2018). "The first record of albanerpetontid amphibians (Amphibia: Albanerpetontidae) from East Asia". PLOS One. 13 (1): e0189767. doi:10.1371/journal.pone.0189767. PMC 5752013. PMID 29298317.
- Stuart-Fox, D.; Moussalli, Adnan; Whiting, Martin J. (2007). "Natural Selection on Social Signals: Signal Efficacy and the Evolution of Chameleon Display Coloration". The American Naturalist. 170 (6): 916–930. doi:10.1086/522835. PMID 18171173.
- Glaw, Frank; Vences, Miguel (1994). A Field Guide to Amphibians and Reptiles of Madagascar (2nd ed.). Köln: Verlags GbR. ISBN 978-3-929449-01-3.
- Ott, M.; Schaeffel, F.; Kirmse, W. (1998). "Binocular vision and accommodation in prey-catching chamaeleons". Comparative Physiology A. 182 (3): 319–330. doi:10.1007/s003590050182.
- Ott, M., and F. Schaeffel. (1995). A negatively powered lens in the chameleon. Nature 373:692-694
- Le Berre and Bartlett, p. 31
- "Chamaeleon News, August 2004". Chameleonnews.com. Archived from the original on 22 January 2008. Retrieved 1 November 2017.CS1 maint: BOT: original-url status unknown (link)
- Higham, T. E.; Anderson, C. V. (2014), "Function and adaptation of chameleons", in Tolley, K. A.; Herrel, A. (eds.), The Biology of Chameleons, Berkeley, CA: University of California Press, pp. 63–83
- Anderson, C. V.; Sheridan, T.; Deban, S. M. (2012). "Scaling of the ballistic tongue apparatus in chameleons". Journal of Morphology. 273 (11): 1214–1226. doi:10.1002/jmor.20053. PMID 22730103.
- Anderson, Christopher V. (2009) Rhampholeon spinosus feeding video. chamaeleonidae.com
- Herrel, A.; Meyers, J. J.; Nishikawa, K. C.; De Vree, F. (2001). "Morphology and histochemistry of the hyolingual apparatus in chameleons". Journal of Morphology. 249 (2): 154–170. doi:10.1002/jmor.1047. PMID 11466743.
- de Groot, J. H.; van Leeuwen, J. L. (2004). "Evidence for an elastic projection mechanism in the chameleon tongue". Proceedings of the Royal Society of London B. 271 (1540): 761–770. doi:10.1098/rspb.2003.2637. PMC 1691657. PMID 15209111.
- Anderson, C. V.; Higham, T. E. (2014), "Chameleon anatomy", in Tolley, K. A.; Herrel, A. (eds.), The Biology of Chameleons, Berkeley, CA: University of California Press, pp. 7–55
- Anderson, C. V.; Deban, S. M. (2010). "Ballistic tongue projection in chameleons maintains high performance at low temperature". Proceedings of the National Academy of Sciences of the United States of America. 107 (12): 5495–5499. Bibcode:2010PNAS..107.5495A. doi:10.1073/pnas.0910778107. PMC 2851764. PMID 20212130.
- Anderson, C. V.; Deban, S. M. (2012). "Thermal effects on motor control and in vitro muscle dynamics of the ballistic tongue apparatus in chameleons". Journal of Experimental Biology. 215 (24): 4345–4357. doi:10.1242/jeb.078881. PMID 23125336.
- Herrel, A.; Meyers, J. J.; Aerts, P.; Nishikawa, K. C. (2000). "The mechanics of prey prehension in chameleons" (PDF). Journal of Experimental Biology. 203 (Pt 21): 3255–3263. PMID 11023845.
- Prötzel, David; Heß, Martin; Scherz, Mark D.; Schwager, Martina; Padje, Anouk van’t; Glaw, Frank (2018-01-15). "Widespread bone-based fluorescence in chameleons". Scientific Reports. 8 (1): 698. Bibcode:2018NatSR...8..698P. doi:10.1038/s41598-017-19070-7. ISSN 2045-2322. PMC 5768862. PMID 29335580.
- Elaina Zachos (2018-01-18). "Chameleon Bones Glow in the Dark, Even Through Skin". National Geographic. Retrieved 2018-08-03.
- "Habitat loss and fragmentation reduce chameleon population in Tanzania". Phys.org. Retrieved 1 November 2017.
- Glaw, Frank; Vences, Miguel (1994). A Field Guide to Amphibians and Reptiles of Madagascar (2nd ed.). Köln: Verlags GbR. ISBN 978-3-929449-01-3.
- "African Rainforest". Jackson's Chameleon. Toronto Zoo. Archived from the original on November 11, 2011. Retrieved January 9, 2012.
- Dever, Jennifer (December 5, 2007). "Common Chameleon" (PDF). usfca.edu. Archived from the original (PDF) on February 3, 2015. Retrieved January 9, 2012.
- "Reptiles and Amphibians: Veiled Chameleon". Smithsonian National Zoological Park. Archived from the original on 2011-12-17. Retrieved January 9, 2012.
- Karsten, K. B.; Ferguson G. W.; Chen T. C.; Holick M. F. (2009). "Panther chameleons, Furcifer pardalis, behaviorally regulate optimal exposure to UV depending on dietary vitamin D3 status". Physiol. Biochem. Zool. 82 (3): 218–25. doi:10.1086/597525. PMID 19335229.
- Le Berre and Bartlett, p. 110
- Le Berre and Bartlett, p. 109
- Sloboda, Michal; Modrý, David (2006). "New species of Choleoeimeria (Apicomplexa: Eimeriidae) from the veiled chameleon, Chamaeleo calyptratus (Sauria: Chamaeleonidae), with taxonomic revision of eimerian coccidia from chameleons". Folia Parasitologica. 53 (2): 91–97. doi:10.14411/fp.2006.012. PMID 16898122.
Bibliography
- Le Berre, François; Bartlett, Richard D. (2009) The Chameleon Handbook. Barron's Educational Series. 3rd Edition. ISBN 0764141422.
Further reading
| Wikimedia Commons has media related to Chamaeleonidae. |
| Wikispecies has information related to Chamaeleonidae |
- Anderson, C. V.; Deban, S. M. (2010). "Ballistic tongue projection in chameleons maintains high performance at low temperature". Proceedings of the National Academy of Sciences of the United States of America. 107 (12): 5495–5499. Bibcode:2010PNAS..107.5495A. doi:10.1073/pnas.0910778107. PMC 2851764. PMID 20212130.
- Anderson, C. V.; Deban, S. M. (2012). "Thermal effects on motor control and in vitro muscle dynamics of the ballistic tongue apparatus in chameleons". Journal of Experimental Biology. 215 (24): 4345–4357. doi:10.1242/jeb.078881. PMID 23125336.
- Anderson, C. V.; Sheridan, T.; Deban, S. M. (2012). "Scaling of the ballistic tongue apparatus in chameleons". Journal of Morphology. 273 (11): 1214–1226. doi:10.1002/jmor.20053. PMID 22730103.
- Davison, Linda J. Chameleons: Their Care and Breeding. Hancock House Publishers, 1997.
- de Groot, J. H.; van Leeuwen, J. L. (2004). "Evidence for an elastic projection mechanism in the chameleon tongue. ". Proceedings of the Royal Society of London B. 271 (1540): 761–770. doi:10.1098/rspb.2003.2637. PMC 1691657. PMID 15209111.
- de Vosjoli, Philippe. Essential Care of Chameleons. Advanced Vivarium Systems, 2004.
- Herrel, A.; Meyers, J. J.; Nishikawa, K. C.; De Vree, F. (2001). "Morphology and histochemistry of the hyolingual apparatus in chameleons". Journal of Morphology. 249 (2): 154–170. doi:10.1002/jmor.1047. PMID 11466743.