Bulky cyclopentadienyl ligands
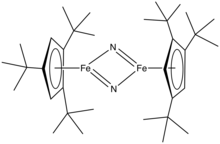
In the area of organometallic chemistry, a bulky cyclopentadienyl ligand is jargon for a ligand of the type C5H5−nRn− where R is larger than n-alkyl and n = 3 or 4. Representative examples are the tetraisopropyl derivative C5H(iPr)4− and the tris tert-butyl derivative 1,2,4-C5H2(tert-Bu)3−. These ligands are so large that their complexes behave differently from the pentamethylcyclopentadienyl analogues. Because they cannot closely approach the metal, these bulky ligands stabilize high spin complexes, e.g. (C5H2(tert-Bu)3)2Fe2I2. These large ligands stabilize highly unsaturated derivatives such as (C5H2(tert-Bu)3)2Fe2N2.
3C5H3.png)
Synthesis and reactions
The (tert-butyl)cyclopentadiene is prepared by alkylation of cyclopentadiene with tert-butyl bromide in the presence of sodium hydride and dibenzo-18-crown-6.[1] The intermediate in this synthesis is di-tert-butylcyclopentadiene. This compound is conveniently prepared by alkylation of cyclobutadiene with tert-butyl bromide under phase-transfer conditions.[2][1]
Illustrative of the unusual complexes made possible with these bulky ligands is molecular iron nitrido complex ((t-Bu)3C5H2)2Fe2N2.[3] In contrast to (C5Me5)2Ir2Cl4, ((t-Bu)3C5H2)IrCl2 is monomeric.[4]
References
- Reiners, Matthias; Ehrlich, Nico; Walter, Marc D. (2018). "Synthesis of 1,3,5-Tri-tert-Butylcyclopenta-1,3-diene and Its Metal Complexes Na{1,2,4-(Me3C)3C5H2} and Mg{η5-1,2,4-(Me3C)3C5H2)2". Inorganic Syntheses. 37: 199. doi:10.1002/9781119477822.ch8.
- Hyster, Todd K. (2015). "1,3 Di-tert-butylcyclopentadiene". Encyclopedia of Reagents for Organic Synthesis: 1–2. doi:10.1002/047084289X.rn01866. ISBN 9780470842898.
- Reiners, Matthias; Maekawa, Miyuki; Daniliuc, Constantin G.; Freytag, Matthias; Jones, Peter G.; White, Peter S.; Hohenberger, Johannes; Sutter, Jörg; Meyer, Karsten; Maron, Laurent; Walter, Marc D. (2017). "Reactivity studies on [Cp′Fe(μ-I)]2: Nitrido-, sulfido- and diselenide iron complexes derived from pseudohalide activation". Chemical Science. 8 (5): 4108–4122. doi:10.1039/C7SC00570A. PMC 6099922. PMID 30155215.
- Shimogawa, Ryuichi; Takao, Toshiro; Suzuki, Hiroharu (2017). "Half-sandwich Cyclopentadienyl Iridium Dichloride Monomer Cp‡IrCl2 (Cp‡ = 1,2,4-tri-tert-butylcyclopentadienyl)". Chemistry Letters. 46 (2): 197–199. doi:10.1246/cl.160937.