Pentamethylcyclopentadienyl iridium dichloride dimer
Pentamethylcyclopentadienyl iridium dichloride is an organometallic compound with the formula [(C5(CH3)5IrCl2)]2, commonly abbreviated [Cp*IrCl2]2 This bright orange air-stable diamagnetic solid is a reagent in organometallic chemistry.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Di-µ-chloro-bis[chloro(pentamethylcyclopentadienyl)iridium(III)] | |
| Other names
Dichloro(pentamethylcyclopentadienyl)iridium(III) | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.205.779 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C20H30Cl4Ir2 | |
| Molar mass | 796.71 g/mol |
| Appearance | orange solid |
| Melting point | >230 °C |
| Dichloromethane, Chloroform | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
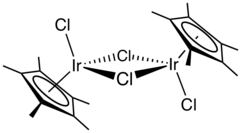
Structure
The compound has C2h symmetry. Each metal is pseudo-octahedral. The terminal and bridging Ir-Cl bonds have the lengths 2.39 and 2.45 Å, respectively. When Cp* is replaced by C5H2(t-Bu)3, the resulting complex is a monomeric, two-legged piano-stool complex. In this mono-Ir complex, the Ir-Cl bond lengths are 2.31 Å.[2]
Preparation, reactions
Pentamethylcyclopentadienyl iridium dichloride dimer was first prepared by the reaction of hydrated iridium trichloride with hexamethyl Dewar benzene.[3] More conveniently, the compound is prepared by the reaction of hydrated iridium trichloride and pentamethylcyclopentadiene in hot methanol, from which the product precipitates[1]
- 2 Cp*H + 2 IrCl3(H2O)3 → [Cp*IrCl2]2 + 2 HCl + 6 H2O
The Ir-μ-Cl bonds are labile and can be cleaved to give a variety of adducts of the general formula Cp*IrCl2L. Such adducts undergo further substitution to afford cations [Cp*IrClL2]+ and [Cp*IrL3]2+. The chloride ligands can also be replaced by other anions such as carboxylates, nitrite, and azide.
Reduction of [Cp*IrCl2]2 in the presence of CO affords [Cp*Ir(CO)2], which can be decarbonylated to give the unsaturated derivative [Cp*Ir(CO)]2.[4] Treatment of [Cp*IrCl2]2 with borohydride under an atmosphere of H2 gives the iridium(V) derivative Cp*IrH4.
[Cp*IrCl2]2 is a precursor to catalysts for the asymmetric transfer hydrogenation of ketones.[5]
References
- White, C.; Yates, A.; Maitlis, P. M. (1992). "(η5-Pentamethylcyclopentadienyl)Rhodium and -Iridium Compounds". Inorg. Synth. 29: 228–234. doi:10.1002/9780470132609.ch53.
- Shimogawa, Ryuichi; Takao, Toshiro; Suzuki, Hiroharu (2017). "Half-sandwich Cyclopentadienyl Iridium Dichloride Monomer Cp‡IrCl2 (Cp‡: 1,2,4-tri-tert-butylcyclopentadienyl)". Chemistry Letters. 46 (2): 197–199. doi:10.1246/cl.160937.
- Kang, Jung W.; Moseley, K.; Maitlis, Peter M. (1969). "Pentamethylcyclopentadienylrhodium and -iridium halides. I. Synthesis and properties". J. Am. Chem. Soc. 91 (22): 5970–5977. doi:10.1021/ja01050a008.
- Ball, R. G.; Graham, W. A. G.; Heinekey, D. M.; Hoyano, J. K.; McMaster, A. D.; Mattson, B. M.; Michel, S. T. (1990). "Synthesis and structure of dicarbonylbis(η-pentamethylcyclopentadienyl)diiridium". Inorg. Chem. 29 (10): 2023–2025. doi:10.1021/ic00335a051.
- Ikariya, T.; Blacker, A. J. (2007). "Asymmetric transfer hydrogenation of ketones with bifunctional transition metal-based molecular catalysts". Acc. Chem. Res. 40 (12): 1300–1308. doi:10.1021/ar700134q. PMID 17960897.