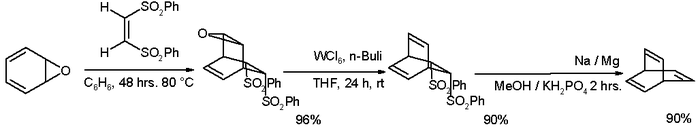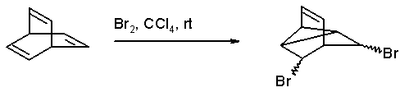Barrelene
Barrelene is a bicyclic organic compound with chemical formula C8H8 and systematic name bicyclo[2.2.2]octa-2,5,7-triene. First synthesized and described by Howard Zimmerman in 1960, the name derives from the resemblance to a barrel, with the staves being three ethylene units attached to two methine groups. It is the formal Diels–Alder adduct of benzene and acetylene. Due to its unusual molecular geometry, the compound is of considerable interest to theoretical chemists.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Bicyclo[2.2.2]octa-2,5,7-triene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H8 | |||
| Molar mass | 104.15 | ||
| Density | 1.013 g/mL | ||
| Boiling point | 153.7 °C (308.7 °F; 426.8 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Iptycenes, with the alkene groups part of an arenes, are related compounds. It is also a starting material for many other organic compounds, such as semibullvalene.
The original Zimmerman, synthesis modified in 1969,[1] starts from coumalic acid:[2]
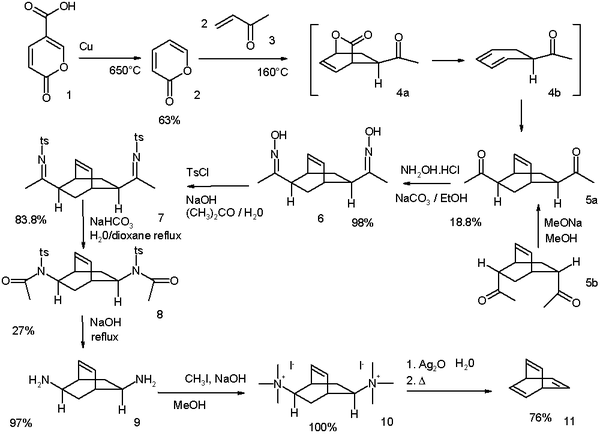 The synthesis of barrelene as reported by Zimmermann in 1969
The synthesis of barrelene as reported by Zimmermann in 1969
Many alternative routes have been devised since then, one of them starting from oxepin:[3][4]
An alternate route that allows synthesis of the parent barrelene system and a variety of substituted barrelenes has also been reported[5]
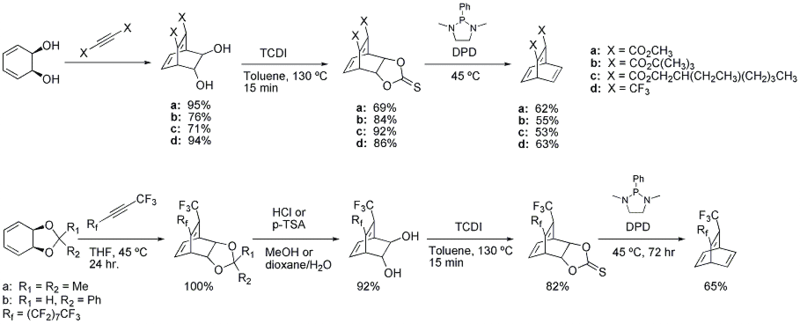
Barrelene reactions
Barrelene is hydrogenated with hydrogen gas and Adams' catalyst in ethanol to the fully saturated bicyclo[2.2.2]-octane. Bromination with bromine in tetrachloromethane gives a di-bromo adduct because a coupling reaction intervenes:
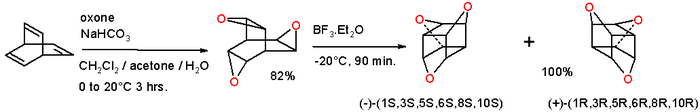
Epoxidation of barrelene with oxone gives the trioxatrishomobarrelene[6] which on rearrangement with boron trifluoride (driving force:relief of strain energy) converts into the trioxatrishomocubane:[7]
This compound can be envisioned as a cubane with three oxygen atoms inserted into three opposite edges or as 9-crown-3 capped by two methine units. The molecule is chiral and the separate enantiomers have been isolated.
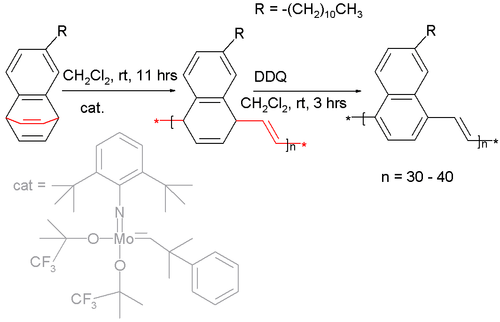
Certain barrelenes have been used as a monomer in a ring opening metathesis polymerization :[8] [9]
The catalyst is a Fischer carbene (a molybdenum bis-(hexafluoro-tert-butoxy) carbene catalyst) and the long alkyl chain attached to the monomer is required for solubility. Oxidation of the polymer with DDQ affords the naphthalene pendant of poly(p-phenylene vinylene).
Isopentane solutions of barrelene undergo photolytic isomerisation when acetone is added as a photosensitizer to produce semibullvalene. Prolonged irradiation results in further isomerisation to form cyclooctatetraene.[10]
References
- Synthesis and physical properties of barrelene, a unique Möbius-like molecule Howard E. Zimmerman, Gary L. Grunewald, Robert M. Paufler, Maynard A. Sherwin J. Am. Chem. Soc.; 1969; 91(9); 2330–2338. doi:10.1021/ja01037a024
- Reaction scheme: decarboxylation of coumalic acid (1) takes place at 650 °C with copper to give α-pyrone (2). The reaction with methyl vinyl ketone (3) is a tandem Diels–Alder/retro-Diels–Alder/Diels–Alder sequence, which yields di-ketone 5 as a mixture of two isomers. It is possible to convert the endo isomer 5b to the exo isomer 5a by an epimerization process through the enol. The ketone groups are converted to oxime groups in 6 by reaction with hydroxylamine and then to the tosylate groups in 7 by reaction with tosyl chloride. A basic Beckmann rearrangement takes the scheme to give amide 8 and its hydrolysis to the di-amine 9 takes place with sodium hydroxide. Finally, a Hofmann elimination through ammonium salt 10 gives the barrelene 11.
- Barrelene, a New Convenient Synthesis Sergio Cossu, Simone Battaggia, and Ottorino De Lucchi J. Org. Chem.; 1997; 62(12) pp 4162–4163; doi:10.1021/jo962267f
- Step one in this reaction between oxepin (one of the possible tautomers) with (Z)-1,2-bis(phenylsulfonyl)ethylene is a Diels–Alder reaction. The reagents for de-epoxidation are tungsten hexachloride and butyllithium. The second elimination reaction takes place with sodium amalgam in Julia olefination style.
- Synthesis of Substituted Bicyclo[2.2.2]octatrienes Michael W. Wagaman, Erika Bellmann, Michèle Cucullu, and Robert H. Grubbs J. Org. Chem.; 1997; 62(26) pp 9076–9082; doi:10.1021/jo971039y
- endo, exo,syn-3,7,10-Trioxapentacyclo[3.3.3.02,4.06,8.09,11]undecane
- 4,7,11-Triheterotrishomocubanes - Propeller-Shaped Highly Symmetrical Chiral Molecules Derived from Barrelene Sergei I. Kozhushkov et al. European Journal of Organic Chemistry Volume 2006, Issue 11 , Pages 2590 - 2600 Abstract
- Synthesis of Poly(1,4-naphthylenevinylenes): Metathesis Polymerization of Benzobarrelenes Lin Pu, Michael W. Wagaman, and Robert H. Grubbs Macromolecules; 1996; 29(4) pp 1138 - 1143; (Article) doi:10.1021/ma9500143
- Synthesis of Organic and Water Soluble Poly(1,4-phenylenevinylenes) Containing Carboxyl Groups: Living Ring-Opening Metathesis Polymerization (ROMP) of 2,3-Dicarboxybarrelenes Michael W. Wagaman, and Robert H. Grubbs Macromolecules; 1997; 30(14) pp 3978–3985; (Article) doi:10.1021/ma9701595
- Zimmerman, H. E.; Grunewald, G. L. (1966). "The Chemistry of Barrelene. III. A Unique Photoisomerization to Semibullvalene". J. Am. Chem. Soc. 88 (1): 183–184. doi:10.1021/ja00953a045.