Coupling reaction
A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon-carbon bond in the product R-R'. The most common type of coupling reaction is the cross coupling reaction.[1][2][3]
Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed cross coupling reactions.[4][5]
Broadly speaking, two types of coupling reactions are recognized:
- Heterocouplings combine two different partners, such as in the Heck reaction of an alkene (RC=CH) and an alkyl halide (R'-X) to give a substituted alkene. Heterocouplings are called cross-couplings.
- Homocouplings couple two identical partners, as in the Glaser coupling of two acetylides (RC≡CH) to form a dialkyne (RC≡C-C≡CR).
Homo-coupling types
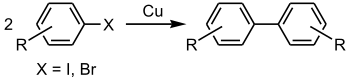
Coupling reactions are illustrated by the famous Ullmann reaction:
| Reaction | Year | Reactant A | Reactant B | Reagent | Remark | ||
|---|---|---|---|---|---|---|---|
| Wurtz reaction | 1855 | R-X | sp3 | R-X | sp3 | Na as reducing agent | |
| Pinacol coupling reaction | 1859 | R-HC=O or R2(C=O) | R-HC=O or R2(C=O) | various metals | requires proton donor | ||
| Glaser coupling | 1869 | RC≡CH | sp | RC≡CH | sp | Cu | O2 as H-acceptor |
| Ullmann reaction | 1901 | Ar-X | sp2 | Ar-X | sp2 | Cu | high temperatures |
Cross-coupling types
An illustrative cross-coupling reaction is the Heck coupling of an alkene and an aryl halide:
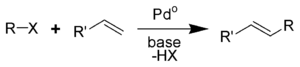
| Reaction | Year | Reactant A | Reactant B | Catalyst | Remark | ||
|---|---|---|---|---|---|---|---|
| Grignard reaction | 1900 | R-MgBr | sp, sp2, sp3 | R-HC=O or R(C=O)R2 | sp2 | not catalytic | |
| Gomberg-Bachmann reaction | 1924 | Ar-H | sp2 | Ar'-N2+X− | sp2 | not catalytic | |
| Cadiot-Chodkiewicz coupling | 1957 | RC≡CH | sp | RC≡CX | sp | Cu | requires base |
| Castro-Stephens coupling | 1963 | RC≡CH | sp | Ar-X | sp2 | Cu | |
| Corey-House synthesis | 1967 | R2CuLi or RMgX | sp3 | R-X | sp2, sp3 | Cu | Cu-catalyzed version by Kochi, 1971 |
| Cassar reaction | 1970 | Alkene | sp2 | R-X | sp3 | Pd | requires base |
| Kumada coupling | 1972 | Ar-MgBr | sp2, sp3 | Ar-X | sp2 | Pd or Ni or Fe | |
| Heck reaction | 1972 | alkene | sp2 | Ar-X | sp2 | Pd or Ni | requires base |
| Sonogashira coupling | 1975 | RC≡CH | sp | R-X | sp3 sp2 | Pd and Cu | requires base |
| Negishi coupling | 1977 | R-Zn-X | sp3, sp2, sp | R-X | sp3 sp2 | Pd or Ni | |
| Stille cross coupling | 1978 | R-SnR3 | sp3, sp2, sp | R-X | sp3 sp2 | Pd | |
| Suzuki reaction | 1979 | R-B(OR)2 | sp2 | R-X | sp3 sp2 | Pd or Ni | requires base |
| Hiyama coupling | 1988 | R-SiR3 | sp2 | R-X | sp3 sp2 | Pd | requires base |
| Buchwald-Hartwig reaction | 1994 | R2N-H | sp3 | R-X | sp2 | Pd | N-C coupling, second generation free amine |
| Fukuyama coupling | 1998 | R-Zn-I | sp3 | RCO(SEt) | sp2 | Pd or Ni[6] | |
| Liebeskind–Srogl coupling | 2000 | R-B(OR)2 | sp3, sp2 | RCO(SEt) Ar-SMe | sp2 | Pd | requires CuTC |
Applications
Coupling reactions are routinely employed in the preparation of pharmaceuticals.[3] Conjugated polymers are prepared using this technology as well.[7]
References
- Organic Synthesis using Transition Metals Rod Bates ISBN 978-1-84127-107-1
- New Trends in Cross-Coupling: Theory and Applications Thomas Colacot (Editor) 2014 ISBN 978-1-84973-896-5
- King, A. O.; Yasuda, N. "Palladium-Catalyzed Cross-Coupling Reactions in the Synthesis of Pharmaceuticals". Organometallics in Process Chemistry. Heidelberg: Springer. pp. 205–245. doi:10.1007/b94551.CS1 maint: multiple names: authors list (link)
- "The Nobel Prize in Chemistry 2010 - Richard F. Heck, Ei-ichi Negishi, Akira Suzuki". NobelPrize.org. 2010-10-06. Retrieved 2010-10-06.
- Johansson Seechurn, Carin C. C.; Kitching, Matthew O.; Colacot, Thomas J.; Snieckus, Victor (2012). "Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize". Angewandte Chemie International Edition. 51 (21): 5062–5085. doi:10.1002/anie.201107017. PMID 22573393.
- Nielsen, Daniel K.; Huang, Chung-Yang (Dennis); Doyle, Abigail G. (2013-08-20). "Directed Nickel-Catalyzed Negishi Cross Coupling of Alkyl Aziridines". Journal of the American Chemical Society. 135 (36): 13605–13609. doi:10.1021/ja4076716. ISSN 0002-7863. PMID 23961769.
- Hartwig, J. F. (2010). Organotransition Metal Chemistry, from Bonding to Catalysis. New York: University Science Books. ISBN 1-891389-53-X.